Immuno-oncology Assays
Immuno-oncology CRO Services
Cellomatics Biosciences is a preclinical Immuno-oncology CRO, offering expertise in robust and reproducible immuno-oncology related assays.
Our immune system is an extraordinary sentinel that protects our body from harmful pathogens such us bacteria and viruses (the innate immunity), produce antibodies against foreign substances and destroy abnormal cells that may evolve into cancer cells (the adaptive immunity).
A balanced immune system can perfectly target and remove cancer cells before its evolution to a detectable tumour. However, an imbalanced immune system response could contribute to tumorigenesis or induce inflammation stimulating cancer cell proliferation and metastasis, leaving these cells growing effectively avoiding our immune system. It was in 2010 that the FDA approved the first immune therapy against prostate cancer, launching de facto the modern immunotherapy.
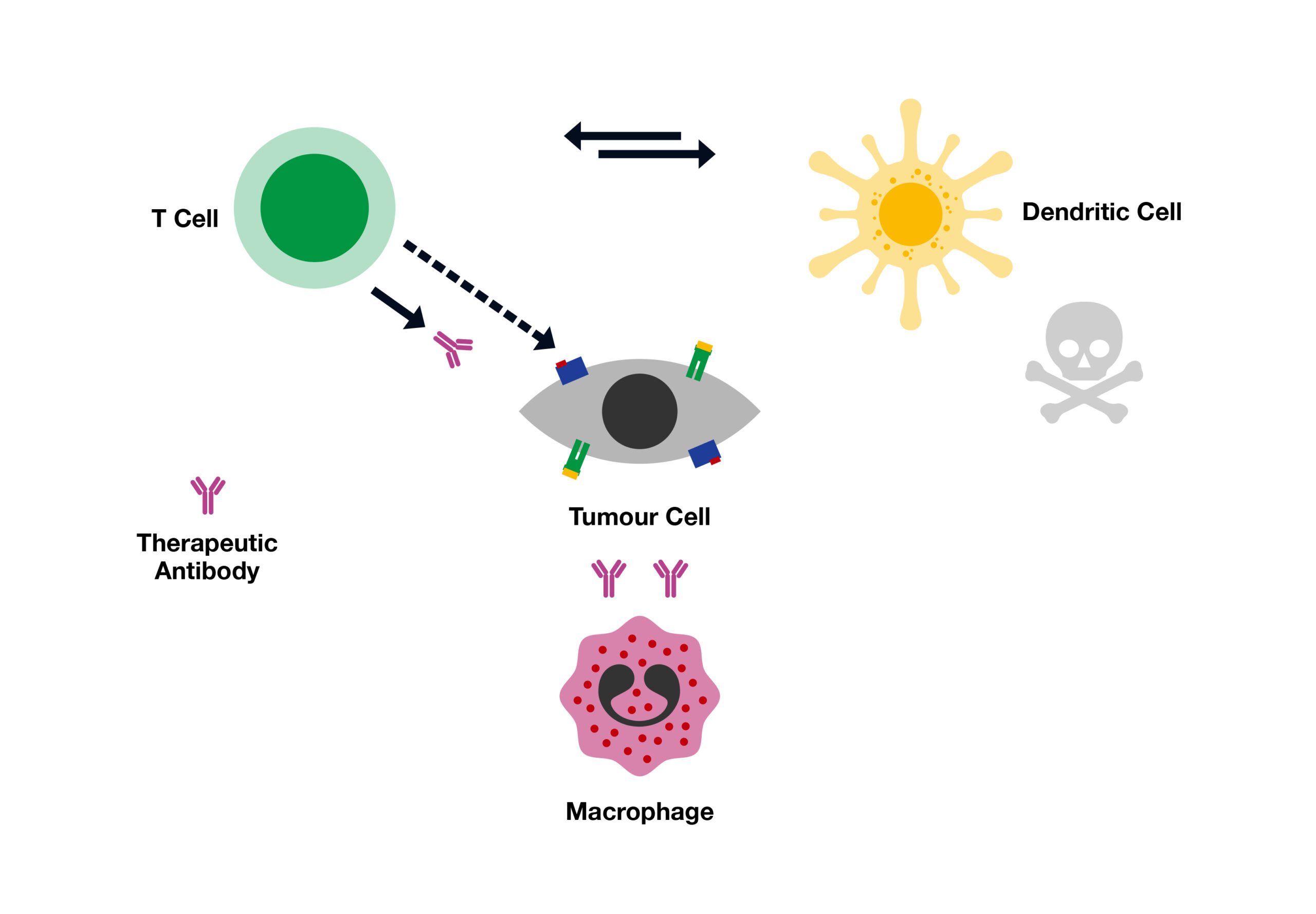
Cancer immunotherapy or immuno-oncology, by definition, represents a specific targeted therapy by using our immune system to fight and destroy cancer cells. As this does not involve surgery or use of radiation or chemotherapy, it is relatively safe to the healthy cells of the body. Importantly, it could be applicable at all stages of the disease, with higher efficiency.
The whole objective of Immunotherapy is to enable our immune system understand differences between healthy and cancer cells and then specifically target the cancer cells. We now have the tools to produce specific substances that stimulate our immune system to recognise and fight specific cancer cells. Monoclonal antibodies, checkpoint inhibitors and cytokines represent a class of immuno-oncology therapies that trigger an immune response to destroy cancer cells.
In conclusion, immunotherapy (or immuno-oncology) has opened a new era in healthcare and indeed we can soon expect some ground-breaking discoveries that will completely erase our current notion of cancer as a life-threatening disease. Having said that, modelling the immune system in vitro to stimulate an effective response to cancer sets a particularly difficult challenge. Potential roles of multiple immune cells, the heterogeneity of tumours and the molecular mechanisms involved mean that multiple advanced assay models are required before moving the most promising immunotherapeutic approaches into clinical trials.
Request more information
For a more in depth look at our immuno-oncology expertise, click the button below to gain access to our immuno-oncology hub, with a detailed look at our data sets and offering.
Request a consultation with Cellomatics Biosciences today
Our experienced team of in vitro laboratory scientists will work with you to understand your project and provide a bespoke project plan with a professional, flexible service and a fast turnaround time.
To request a consultation where we can discuss your exact requirements, please contact Cellomatics Biosciences.