Inflammation Assays
Inflammation CRO Services
Cellomatics Biosciences is a preclinical Inflammation CRO, providing physiologically relevant in vitro inflammatory disease models to investigate the efficacy and/or toxicity of test compounds. These assays can be customised to suit the client’s requirements, with options for bespoke assays with multiple end-point measurements.
Inflammation is a natural defence process initiated against intruders like foreign bodies, irritant molecules or pathogens (like bacteria and viruses).
In response to this aggression, our body unleashes the neutrophils, a type of white blood cell which move toward the affected area through the enlargement of the small blood vessels, releasing specific molecules (like cytokines, reactive oxygen species and proteases) defending our body. This process is called acute inflammation. This may last for few days or persist for few weeks; in this case is called sub-acute inflammation.
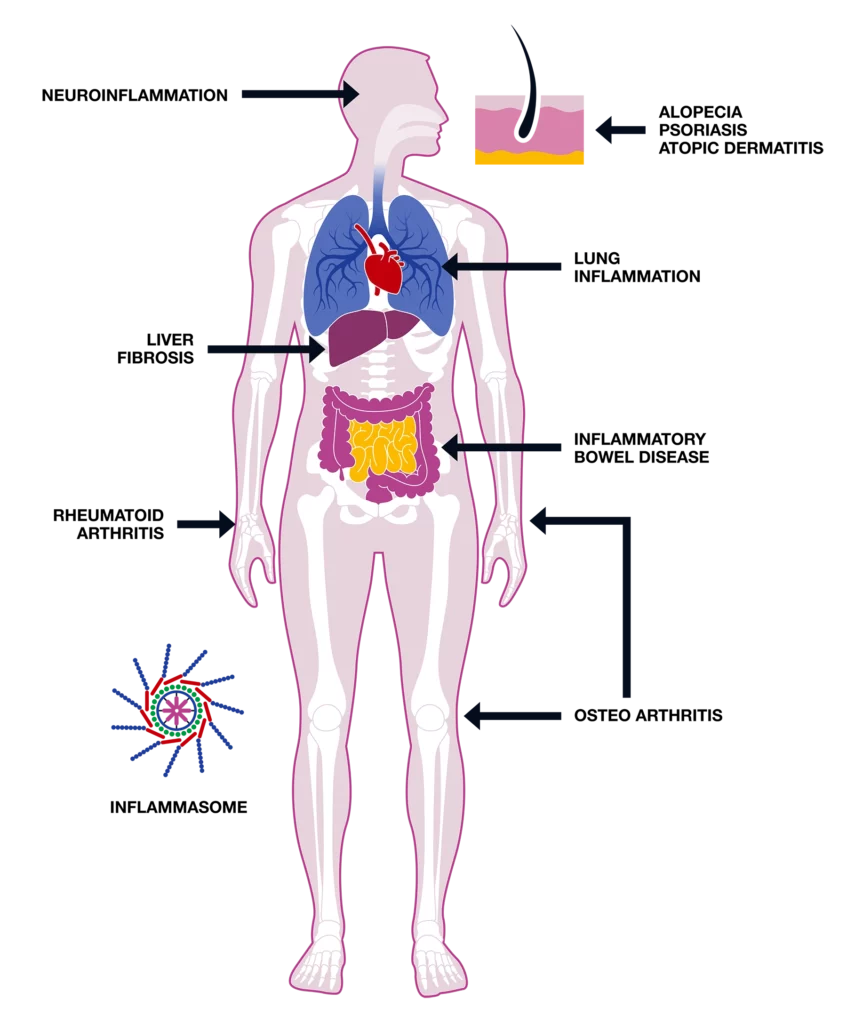
Inflammation is a controlled process and should disappear as soon as the harmful substance or the pathogen is removed. Persistence of this defence mechanism for months or even years, called chronic inflammation, could result in tissue damage producing severe conditions like asthma, chronic obstructive pulmonary disease, atopic dermatitis, psoriasis, osteoarthritis and inflammatory bowel disease.
Request more information
For a more in depth look at our Inflammation expertise, click the button below to gain access to our Inflammation Hub with a detailed look at our data sets and offering.

Request a consultation with Cellomatics Biosciences today
Our experienced team of in vitro laboratory scientists will work with you to understand your project and provide a bespoke project plan with a professional, flexible service and a fast turnaround time.
To request a consultation where we can discuss your exact requirements, please contact Cellomatics Biosciences.







