Oncology CRO Services
Cellomatics Biosciences is a CRO providing expertise in supporting preclinical oncology projects. Alongside the ongoing development of disease models, our team of oncology specialists routinely perform high throughput drug screens, followed by a wide range of assays to measure several biological responses that complement the hallmarks of cancer.
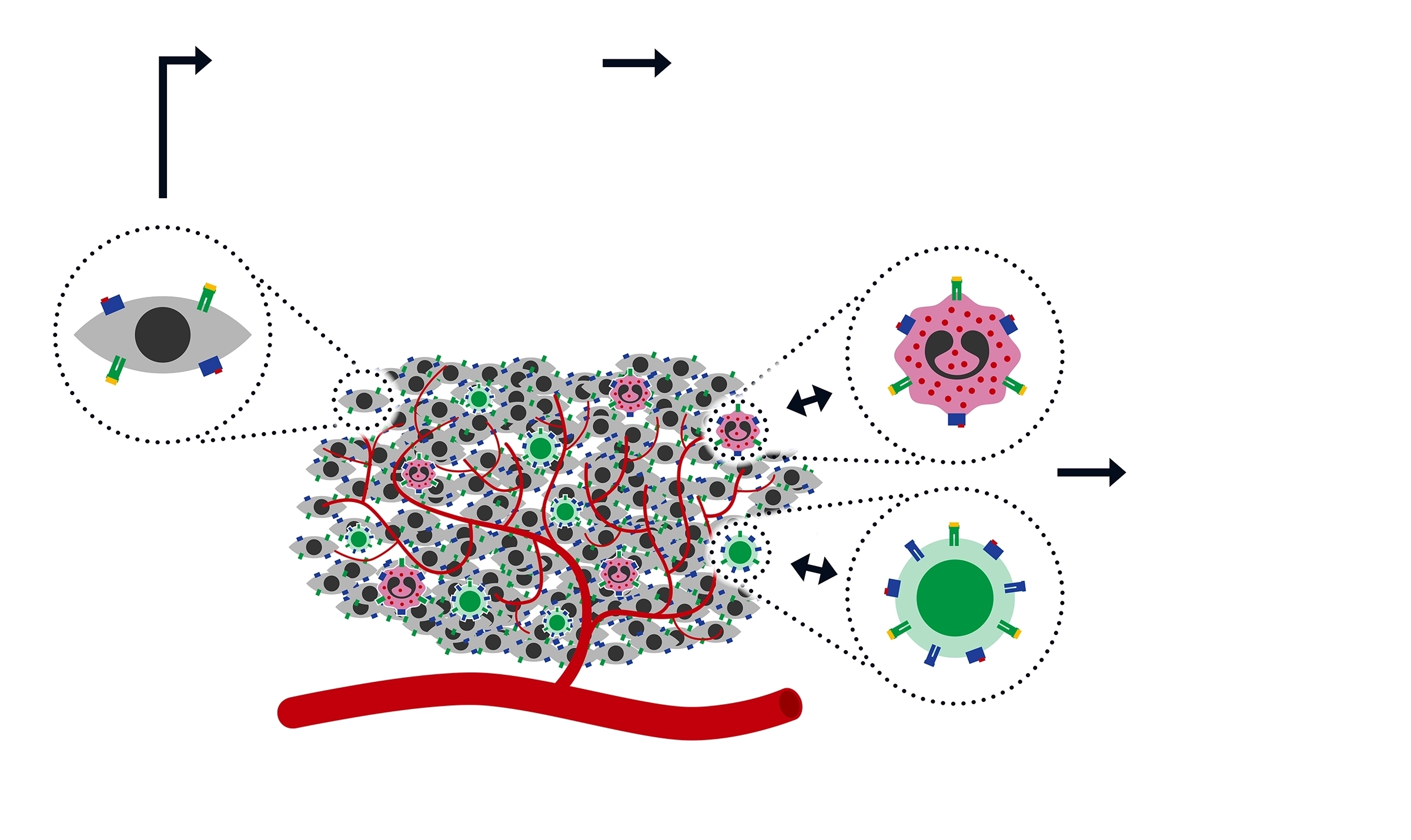
Neoplastic growth can arise from a wide range of cell types, creating a spectrum of disease presentations that vary in background pathology and hence treatment approaches.
The development of cancer arises from a dysregulation in cellular proliferation, which commonly arises from inappropriate responses to external stimuli. Discovery of mutations in key oncogenes and tumour suppressor genes has been pivotal in characterising biological signatures that favour oncogenesis. For instance, many cancers harbour activating mutations in components of several survival pathways (e.g. PI3K and MAPK pathways) leading to constitutive signalling. Downstream effects include increased production of growth factors for inter and intracellular interactions, signalling cross talk and loss of response to inhibitory stimuli. The acquisition of cancer hallmarks, as outlined by Hanahan and Weinberg (2011), has been the foundation of studying cancer biology and for determining optimal treatment approaches. For years, therapeutic regimes have been developed to target the following oncogenic drivers:
- Sustaining proliferative signalling
- Resisting cell death
- Inducing angiogenesis
- Enabling replicative immortality
- Activating invasion and metastasis
- Evading growth suppressors
Request a consultation with Cellomatics Biosciences today
Our experienced team of in vitro laboratory scientists will work with you to understand your project and provide a bespoke project plan with a professional, flexible service and a fast turnaround time.
To request a consultation where we can discuss your exact requirements, please contact Cellomatics Biosciences.