Roboh Borke is a Bioassay Research Scientist at Cellomatics. In this blog, she discusses how, as an Inflammation CRO, Cellomatics is developing co-culture models to study Inflammatory Bowel Disease, with the objective to investigate the effects of interaction between intestinal epithelial cells and immune cells (cell-cell cross talk) in the pathogenesis of the disease.
About inflammatory bowel disease
Inflammatory bowel disease (IBD) is a chronic inflammatory disease of the gastrointestinal tract, encompassing Crohn’s disease (CD), ulcerative colitis (UC), and other conditions. The inflammation of the intestinal mucosa in IBD is characterised by episodes of abdominal pain, diarrhoea, blood in stools and weight loss-this is associated with the influx of neutrophils and macrophages that produce cytokines, proteolytic enzymes, and free radicals, resulting in inflammation and ulceration 1. Leading inflammation CRO, Cellomatics, explore in vitro models to further study IBD.
According to Pasvol et al (2020) and Alatab et al (2020), approximately 2.5-3 million and 1.5 million people in Europe and North America respectively are affected by IBD, with an estimated direct healthcare cost of 4.6–5.6 billion Euros/year2,3.
The therapies available for the treatment of IBD include steroids, immunosuppressive and anti-inflammatory drugs which have variable efficacy, as in most cases, the side effects outweigh the benefits4,5. In a systematic review, out of 3138 patients treated with IBD drugs, 2129 (67.8%) presented with one or several drug-related side effects necessitating drug cessation6. There remains a need for continuous research employing both in vitro strategies and other models in drug discovery.
IBD research
Over the years, research efforts in IBD have been directed towards the epithelium as, epithelial cells have been shown to play a critical role in inflammation6. The epithelial cell lines commonly employed include the colonic adenocarcinoma cell line Caco-2, which when differentiated, expresses several morphological and functional features (formation of tight junctions, secretion of mucin and cytokines) of small intestinal enterocytes. T84 and HT-29 cell lines which can have a goblet cell-like phenotype are also commonly used7. The application of immortalised cell lines remains favourable due to their easy accessibility, handling, and maintenance. Permeability and drug absorption studies using monocultures of these cells are frequently performed. For instance, Caco-2 cells grown on transwell membranes for 21 days to induce differentiation and further challenged with lipoteichoic acid to induce an inflammatory response were used to study the anti-inflammatory effects of the plant Maytenus ilicifoli. Treatment with this plant extract prevented the secretion of IL-8 in the presence of an inflammatory challenge8. HT-29 cells have also been cultured on 12-well plates for immunomodulatory studies of Ulcerative Colitis and Crohn’s Disease9. Although the use of these single cell culture models have provided insights into physiological events, the major disadvantage on using such models is that they do not reflect the actual in vivo conditions as cells do not occur as a single type in vivo. Thus, the need for the development of new pre-clinical models which better reflect in vivo conditions and can predict the effects of an experimental therapeutic compound is crucial.
Primary cultures of human colonic epithelial cells obtained from endoscopic biopsies have also been suggested in the study of inflammatory diseases10; however, the use of freshly isolated cells is limited by their poor survival rate and inability to form tight monolayers.
More recently, 3D culture models have been developed to study the pathogenesis of IBD. For example, intestinal organoids, which are 3D structures derived from either pluripotent stem cells (iPSCs) or primary intestinal crypt stem cells, are capable of spontaneous self-organisation and differentiation, producing functional cell types and functioning similarly to the source tissue when grown on a suitable matrix (Matrigel) and media11. Although these organoids can differentiate into cell lines found in the gut, including mature enterocytes, Paneth cells, goblet cells, enteroendocrine, and tuft cells, they however lack functional microbial and immune cell components11,12. An inclusion of these would provide a more physiologically relevant model of the gastrointestinal mucosal environment. Bioengineering techniques are also under development. 3D printing and laser ablation allow the creation of scaffolds which recreate the intestinal topography. These can then be directly seeded with epithelial organoids or used as moulds to create hydrogel-based porous copies which reproduce the microanatomy of the gut13. Although promising, they are more costly and less accessible when compared to 2D models. The Matrigel and other matrices in which the organoids are cultured are expensive and difficult to manipulate. Lastly, access to human tissue for generation of primary organoid cultures can be limited due to legal regulations relating to the source of stem cells for organoid creation, informed consent, and privacy of cell donors.
Inflammation CRO, Cellomatics provide their expertise
At Cellomatics, we have developed and validated an in vitro 2D co-culture model of inflammation using differentiated Caco-2 cells and macrophages (differentiated THP-1 cells) that can be manipulated to mimic the intestine in either healthy or inflamed states. These two states differ as in the latter, an inflammatory phenotype is achieved by the introduction of an inflammatory stimulus which leads to the production of pro-inflammatory markers. Therefore, we can screen pharmacological reagents supplied by clients in a model which mimics the physiology of inflammation. Here, Caco-2 cells are grown and differentiated on transwells. We can then introduce THP-1 cells which have been treated with phorbol 12-myristate-13-acetate (inducing a macrophage-like morphology) into co-culture along with the differentiated Caco-2 cells. This innovative co-culture model not only better replicates the in vivo conditions but can also be used to study cell-cell interactions, pathophysiological reactions occurring in the intestine during inflammation (e.g., altered epithelial barrier and transport function), immune-mediated tissue destruction, and factors that may influence cell death and necrosis. The beauty of the Caco-2 cells is that during differentiation, they form polarised monolayers with tight junctions similarly to how enterocytes behave in vivo. Using our ImageXpress PICO Automated Cell Imaging system, we can assess the potential damage and disruption of the tight junction proteins caused by inflammation (figure 1).
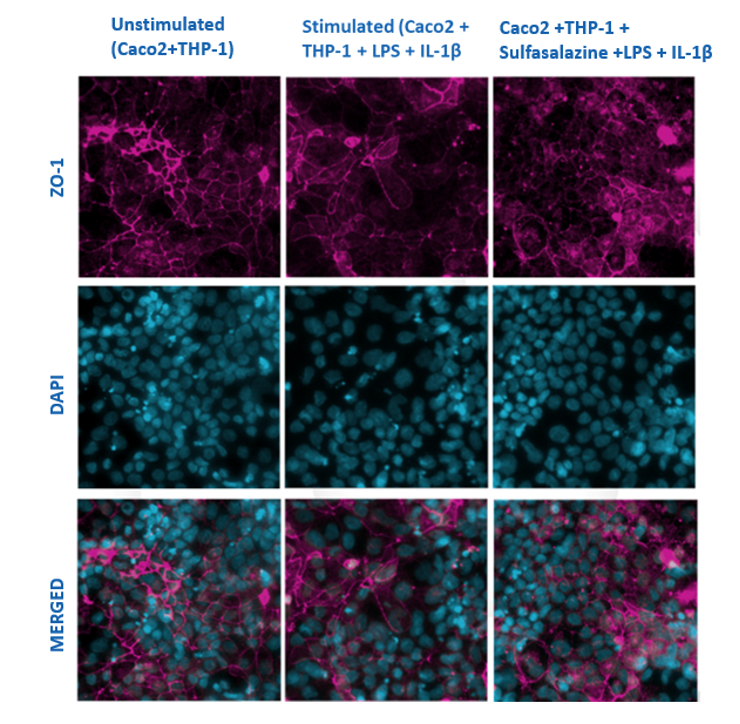

Figure 1
Caco-2 cells (differentiated for 21 days) and PMA differentiated THP-1 cells were grown in co-culture and stimulated with LPS and IL-1βfor 48hrs in the presence or absence of Sulfasalazine. The monolayers were stained after 48hrs for tight junction proteins such as ZO-1 (pink) and with DAPI (blue– nucleus). The images show a reduction in the ZO-1 protein in the stimulated cells compared to the well-defined protein structure seen in the unstimulated cells. Prior treatment with sulfasalazine before stimulation prevented damage to the tight junction protein when compared to the stimulated cells. The images were captured using ImageXpress Pico Imager at a x20 magnification.
In addition to protein localisation, another parameter that we can look at using our model is the Transepithelial electrical resistance (TEER). TEER is the measurement of electrical resistance across a cellular monolayer. During differentiation, the Caco-2 cells form a monolayer on the transwell membrane which has a high electrical resistance, and significant changes can easily be determined under experimental conditions. As can be seen in figure 2 below, using our TEER EVOM3 meter, changes in the TEER can be monitored, measured, and plotted against time in cultures exposed to test compounds. This can be used to assess potential repair of a damaged epithelium or the prevention of damage by compounds supplied by the client.
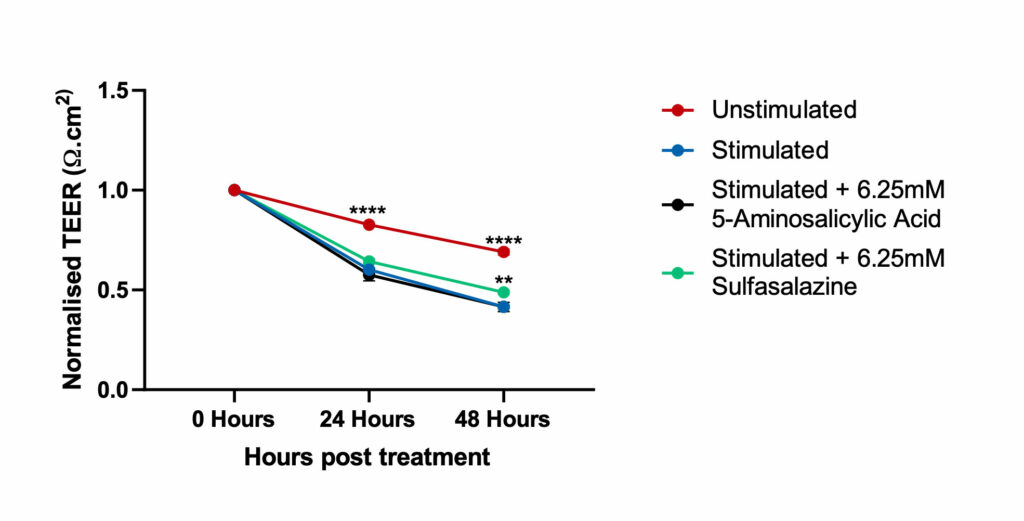

Figure 2
Caco-2 cells differentiated for 21 days and PMA differentiated THP-1 cells were grown in co-culture and stimulated with LPS and IL-1β for 48hrs to induce an Inflammatory Bowel Disease phenotype in the presence or absence of Sulfasalazine and 5-Aminosalicylic Acid. The TEER reported for all the conditions (compounds and time of treatment) were normalised to the basal TEER reading (as reported on day 21 of differentiation, prior to treatment) for each of the transwells. This data was analysed for both compounds using Two-way ANOVA followed by Dunnett’s multiple comparisons test [95% CI of diff.] (*p<0.05, **p<0.01,****p<0.0001). For each time point, all the conditions are compared to the Stimulated control. The co-culture model treated with Sulfasalazine, was significantly different at 48hours and showed an improvement in TEER values when compared to the stimulated co-culture model indicating repair to the epithelium
Note: Unstimulated = Co-culture (Caco-2 +THP-1) alone, Stimulated= Co-culture (Caco-2 +THP-1) + LPS + IL-1β.
Using our Luminex MAGPIX® System, we are able to perform multiplexed immunoassay where we can detect multiple analytes in a single sample. This is advantageous as it reduces cost and time and is highly sensitive and reproducible. Stimulation of the co-culture model with inflammatory stimuli (lipopolysaccharide and IL1-β) induced expression of pro-inflammatory cytokines (detected using the MAGPIX system), which have been known to drive intestinal inflammation (Figure 3). The imbalance of both pro-inflammatory and anti-inflammatory cytokines in IBD not only delays the resolution of inflammation but also leads to disease perpetuation and tissue destruction14.


Figure 3
Pro-inflammatory marker suppression by Sulfasalazine and 5-Aminosalicylic Acid. Significantly increased levels of pro-inflammatory markers were observed after stimulation of the differentiated Caco-2 monolayers with IL-1β and LPS when compared to unstimulated control. The levels of these markers were suppressed when the Caco-2 monolayers were pre-treated with both compounds. Data was analyzed using Ordinary One-way ANOVA followed by Dunnett’s multiple comparisons test [95% CI of diff.] (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns= not significant). All the conditions were compared to the stimulated control for each of the markers and showed significant differences. However, the levels for 5-Aminosalicylic Acid for TNF- alpha and Sulfasalazine and 5-Aminosalicylic Acid for MCP-1 were not significant (ns). Note: Unstimulated = Co-culture (Caco-2 +THP-1), Stimulated= Co-culture (Caco-2 +THP-1) + LPS + IL-1β.
Lactate Dehydrogenase (LDH) release is another parameter we can look at in this model. LDH is a soluble cytoplasmic enzyme that is present in almost all cells and is released into extracellular space when the plasma membrane is damaged. Detecting enzyme function is performed biochemically and increased values can demonstrate possible damage to the cell caused by inflammation or test compounds. In figure 4 below, we observed no significant LDH release when comparing our test compounds to the unstimulated control. showing that the test compounds had no negative effect on the cells. In comparison, treatment with {(+ve control)} resulted in a significant increase in detection of LDH activity.
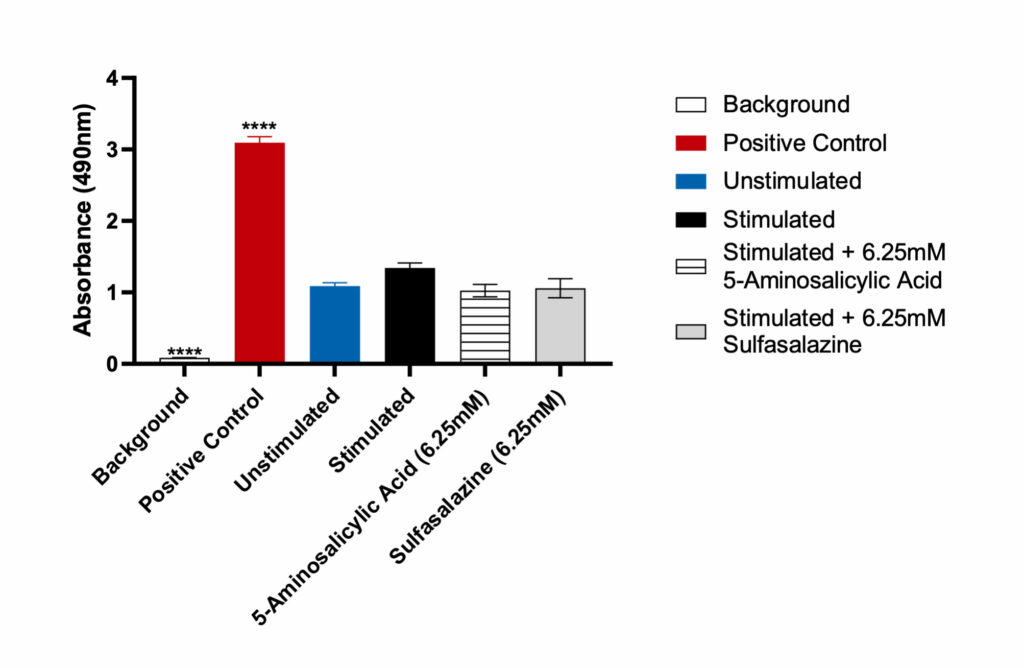

Figure 4
Lactate Dehydrogenase (LDH) release by Caco-2 cells. Data was analyzed using Ordinary One-way ANOVA followed by Dunnett’s multiple comparisons test [95% CI of diff.] (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns= not significant). The LDH positive control, provided with the assay, generated significant OD 450 values (p<0.0001) compared to the unstimulated control. Both Sulfasalazine and 5-Aminosalicylic Acid showed no significant LDH release when compared to the unstimulated control.
Support from experts at inflammation CRO, Cellomatics
Cellomatics has developed a model for studying IBD which can be used to screen and assess the functions of potential compounds of interest. It was developed to be more representative of IBD in vivo by the inclusion of THP-1 cells and is available to clients for testing purposes.
If this model would be of interest to you, then please do not hesitate to contact us for more information.
References
1. Guan Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. Journal of Immunology Research. 2019;2019.
2. Pasvol TJ, Horsfall L, Bloom S, Segal AW, Sabin C, Field N, et al. Incidence and prevalence of inflammatory bowel disease in UK primary care: A population-based cohort study. BMJ Open. 2020 Jul 19;10(7).
3. Alatab S, Sepanlou SG, Ikuta K, Vahedi H, Bisignano C, Safiri S, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet Gastroenterology and Hepatology. 2020 Jan 1;5(1):17–30.
4. Burger D, Travis S. Conventional medical management of inflammatory bowel disease. Gastroenterology. 2011;140(6):1827-1837.e2.
5. Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. Vol. 12, Journal of medicine and life. NLM (Medline); 2019. p. 113–22.
6. Godat S, Fournier N, Safroneeva E, Juillerat P, Nydegger A, Straumann A, et al. Frequency and type of drug-related side effects necessitating treatment discontinuation in the Swiss Inflammatory Bowel Disease Cohort. European Journal of Gastroenterology and Hepatology. 2018;30(6):612–20.
7. Randall-Demllo S, Chieppa M, Eri R. Intestinal epithelium and autophagy: Partners in gut homeostasis. Vol. 4, Frontiers in Immunology. 2013.
8. Liévin-Le Moal V, Servin AL. Pathogenesis of Human Enterovirulent Bacteria: Lessons from Cultured, Fully Differentiated Human Colon Cancer Cell Lines. Microbiology and Molecular Biology Reviews. 2013 Sep;77(3):380–439.
9. Wonfor R, Natoli M, Parveen I, Beckman M, Nash R, Nash D. Anti-inflammatory properties of an extract of M. ilicifolia in the human intestinal epithelial Caco-2 cell line. Journal of Ethnopharmacology. 2017 Sep 14;209:283–7.
10. Østvik AE, Svendsen TD, van Beelen Granlund A, Doseth B, Skovdahl HK, Bakke I, et al. Intestinal epithelial cells express immunomodulatory ISG15 during active ulcerative colitis and Crohn’s disease. Journal of Crohn’s and Colitis. 2020;14(7):920–34.
11. Medical Journal D, Med D, Pedersen IG. DOCTOR OF MEDICAL SCIENCE DANISH MEDICAL JOURNAL The 7 original papers are. Vol. 62, B4973 Autoimmunity. 2015.
12. O’Connell L, Winter DC, Aherne CM. The Role of Organoids as a Novel Platform for Modeling of Inflammatory Bowel Disease. Vol. 9, Frontiers in Pediatrics. Frontiers Media S.A.; 2021.
13. Angus HCK, Butt AG, Schultz M, Kemp RA. Intestinal Organoids as a Tool for Inflammatory Bowel Disease Research. Vol. 6, Frontiers in Medicine. Frontiers Media S.A.; 2020.
14. Kim GA, Spence JR, Takayama S. Bioengineering for intestinal organoid cultures. Vol. 47, Current Opinion in Biotechnology. Elsevier Ltd; 2017. p. 51–8.
15. Neurath MF. Cytokines in inflammatory bowel disease. Vol. 14, Nature Reviews Immunology. Nature Publishing Group; 2014. p. 329–42.






