Cellomatics Biosciences’ Senior Bioassay Scientist Stephanie Holliday introduces INNOBEX™ – a new in vitro model of the blood brain barrier developed in-house here at Cellomatics.
The blood brain barrier
The blood brain barrier (BBB) is key in preventing hazardous substances passing between the bloodstream and the brain, in order to protect the central nervous system and maintain the balance of the interstitial fluid1. In vivo, the BBB is made up of microvascular endothelial cells (Human Brain MVEC or HBMVEC), supported by pericytes, which are fibroblast-like cells that wrap around the endothelial cells, and astrocytes, which are a type of glial cell that regulates blood flow. The blood brain barrier works by modulating the passage of molecules between the blood and the brain. The density of pericytes in the brain is much higher than in other tissues, aiding in reducing the permeability of the BBB2. The end-feet of astrocytes form an additional layer of cells, allowing a quick response for the neural capillaries to contract or dilate. The neural capillaries are made up of MVEC Endothelial cells in the BBB are held together by tight junctions, which create a polarizing barrier between the luminal and abluminal compartments. The tight junctions have a size selective permeability that allows the control of molecules passing between the blood and brain2,3.
In vitro models of the blood brain barrier which are representative of the in vivo BBB are highly sought after, given its role in disease. Pathology of the BBB is important in neurodegenerative diseases such as dementia and Parkinson’s disease1. In dementia, vascular damage to the BBB leads to decreased permeability, and accumulation of Amyloid β proteins. The Amyloid β peptides have neurotoxic properties that lead to dementia4. Furthermore, the BBB can play a role in infective diseases such as Meningitis5. Understanding the function of the BBB in these diseases could lead to increased treatment options and improved quality of life for the patients.
Challenges and limitations of existing BBB models
Over the years, several companies have designed different models to represent the BBB in vitro. These range from simple one cell type models to complex organ on a chip or microfluidic structures. Single cell models are typically comprised of a culture of brain endothelial cells, grown in transwells or in a multiwell plate. This is limiting as the BBB is a highly integrated combination of different cell types, which interact to protect the central nervous system. Using a single cell type alone means that the cells will not receive feedback from the other cell types surrounding the vasculature. It also means that such a tight barrier may not be formed, as only a single layer of cells is present compared with the three in our model. In contrast, other, more complex models are limited by their expense and low throughput6. Microfluidic models for example take much optimisation and therefore are expensive to make. They are also less suitable for high throughput projects. In vivo animal models can be used, but these are unsuitable for some studies where the mode of action is specific to humans7.
Cellomatics Biosciences’ model
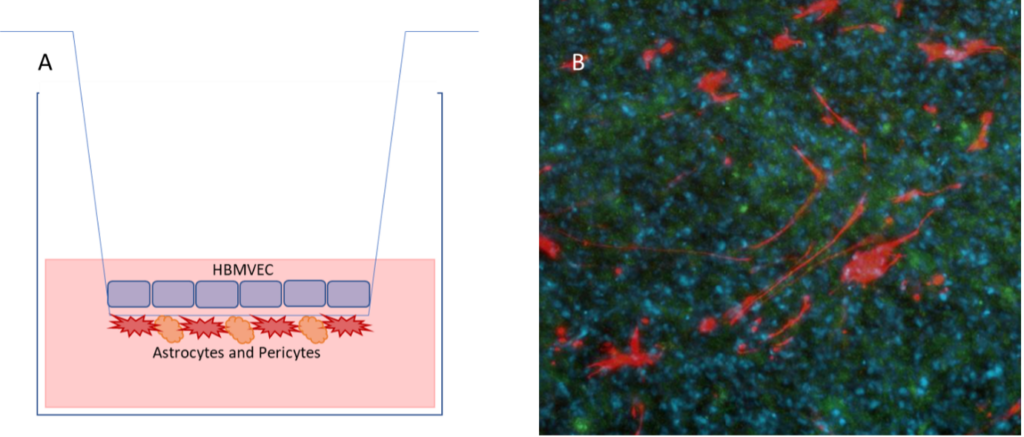

Figure 1: The blood brain barrier model. (A) Astrocytes and pericytes are grown in coculture on the basal side of the transwell, with brain endothelial cells on the apical side. (B) Immunofluorescence demonstrating the different cell types. Astrocytes are detected by GFAP (red), and brain endothelial cells by Smooth muscle actin (green). DAPI (blue) is used to detect nuclei.
The Cellomatics blood brain barrier model, INNOBEX™ was created as a solution for the problems described above. As the model is freshly prepared in-house, we ensure that the cells are healthy and the model is used only once it has passed our stringent quality control measures. INNOBEX is comprised of three primary cell types; endothelial cells (HBMVEC), pericytes and astrocytes. The cells are added to Transwell inserts to represent the blood brain barrier. Astrocytes and pericytes are grown on the basal side of the transwell, which represents the brain, and endothelial cells are grown on the apical side, representing the blood8. Therefore, if a substance is added to the apical / blood side of the transwell, if it is later detectable in the basal / brain side, the substance is able to pass through the blood brain barrier. Other drugs or treatments may be added to increase or decrease the permeability of the blood brain barrier. This happens when the treatment acts to disrupt the tight junctions formed between endothelial cells in the blood vasculature.
Assay applications for the BBB
Permeability assays: TEER and FITC Dextran can be used as stand-alone assays or together to assess the permeability of the BBB. This can be used to determine if a drug is acting on the cells of the BBB to increase or decrease the permeability. TEER (transendothelial electrical resistance) is used to measure the resistance across a barrier9. The higher the TEER, the higher the barrier integrity of the BBB, representing a stronger barrier between the blood and the brain . FITC Dextran permeability assay supports TEER, as an increased fluorescence signal in the media relates to an increase in permeability. In Figure 2 we have used TNFα10 to induce a break in the BBB, whereas other drugs such as Dexamethasone can be used to maintain the tight junctions.
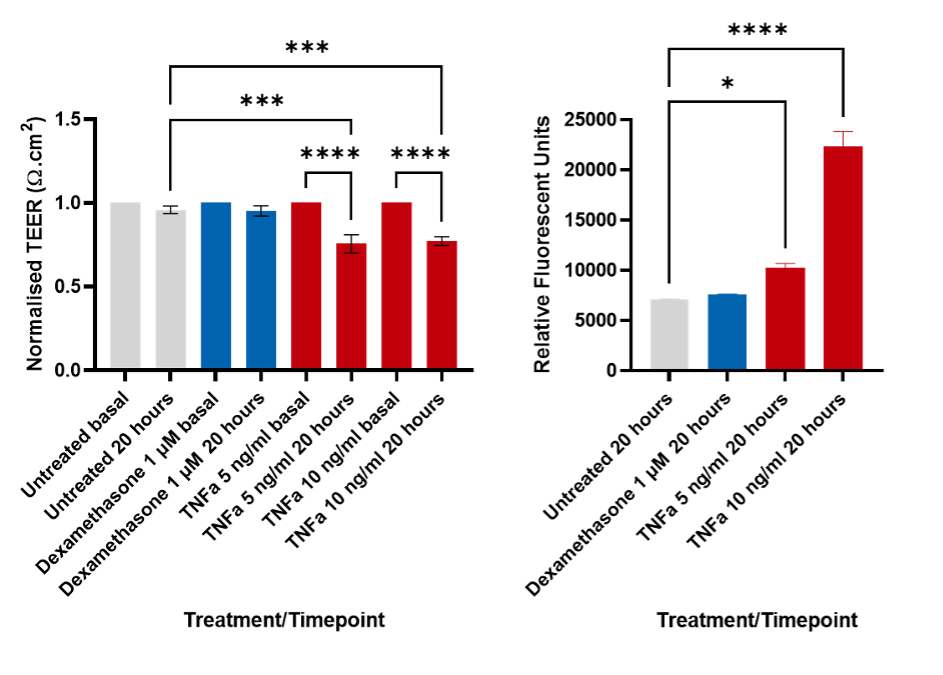

Figure 2: TEER and FITC-dextran assays. A decrease in the TEER correlates with an increase in FITC-Dextran. This demonstrates the permeability of the BBB. TEER data analysed using 1-way ANOVA with Sidak’s multiple comparisons test, FITC-Dextran data were analysed using 1-way ANOVA with Dunnett’s multiple comparison test. * p < 0.05, *** p < 0.001, **** p < 0.0001.
Other assay applications for INNOBEX™ include immunofluorescence (as seen in figure 1) or immunohistochemistry. This would allow clients to assess protein interactions or distribution of cells within the BBB. INNOBEX™ can also be used to study peptide-efflux, important in dementia to understand the build-up of Amyloid β peptides in the brain10. These studies can be performed in conjunction with cytokine analysis, using our Luminex MAGPIX® System to perform multiplex immunoassays where we can detect multiple analytes in a single sample, to see how treatment effects the cytokines on the blood or brain side of the barrier.
To discuss INNOBEX™ and its potential applications for your project, contact us here.
References
- Blood–brain barrier breakdown in Alzheimer’s disease and other neurodegenerative disorders – PMC (nih.gov)
- The Blood–Brain Barrier – PMC (nih.gov)
- Claudins and epithelial paracellular transport – PubMed (nih.gov)
- Neurovascular Dysfunction and Neurodegeneration in Dementia and Alzheimer’s disease – PMC (nih.gov)
- How Pathogens Penetrate the Blood-Brain Barrier (asm.org)
- Benchmarking in vitro tissue-engineered blood–brain barrier models | Fluids and Barriers of the CNS | Full Text (biomedcentral.com)
- Advances in Microfluidic Blood–Brain Barrier (BBB) Models: Trends in Biotechnology (cell.com)
- Frontiers | A Novel Transwell Blood Brain Barrier Model Using Primary Human Cells (frontiersin.org)
- TEER measurement techniques for in vitro barrier model systems – PMC (nih.gov)
- IJMS | Free Full-Text | TNF-α and IL-1β Modulate Blood-Brain Barrier Permeability and Decrease Amyloid-β Peptide Efflux in a Human Blood-Brain Barrier Model (mdpi.com)






