To mark Parkinson’s Awareness Month our Senior Bioassay Scientist Dr Gareth Edwards delves into the role of neuroinflammation and its contribution to the global increase in dementia and associated neurodegenerative neuropathies.
Inflammation plays a vital role in the response to many diseases: exposure to stimuli initiates a signalling cascade which amplifies downstream responses, leading to a disease phenotype. Cellomatics Biosciences boasts a strong portfolio of research in the field of inflammation, successfully delivering in-house validated assays for numerous external client projects.
Neuroinflammation has gained considerable traction as a significant contributor to the sharp global increase in dementia and associated neurodegenerative neuropathies (Dias-Carvalho et al., 2024). Indeed, multiple risk factors identified in the development of Alzheimer’s Disease share neuroinflammation as a central component (Weaver, 2023). Therefore, anti-inflammatory compounds may provide a novel therapeutic approach in the treatment of neuroinflammation, thereby preventing neurodegeneration (Sharif et al., 2024; Zheng et al., 2023).
The landscape of neuroinflammation is inherently complex, where signalling pathways display significant overlap, feedback, and crosstalk. Such intricate systems require more complex assays to effectively determine robust measures for potential therapeutics. To meet this need, Cellomatics Biosciences. are developing a multiplexed panel of assays, combining off-the-shelf kits to generate complementary readouts from single samples. This platform provides early stage, cost-effective, high-throughput therapeutic profiling for neuroinflammatory processes in dementia (e.g., Parkinsons Disease, PD, or Alzheimer’s Disease, AD).
This multiplexed approach aims to capture both the direct targeted and indirect reciprocal effects of test therapeutics. For instance, one can confirm that a potential therapeutic effectively targets the amyloidogenic pathway in AD, whilst also gaining knowledge on how it may simultaneously influence the non-amyloidogenic pathway. Moreover, multiple targets can be assayed in a single sample, allowing us to derive greater biological knowledge from a simple 2D culture model.
Importantly, this approach is not limited to 2D culture models, but can be integrated into 3D platforms such as spheroids, or co-cultures of neurons and microglia.
Parkinson’s Disease
Parkinson’s disease is a form of dementia which initially targets motor-specific capabilities (Moustafa et al., 2016). It is characterised by the death of dopaminergic cells in a region of the brain called the substantia nigra, with neuroinflammation thought to play a role in defining the disease phenotype (Grotemeyer et al., 2022). An effective in vitro model of PD should induce the disease phenotype by targeting the dopaminergic cells (Figure 1). One of the simplest existing models utilises dopaminergic differentiation of the neuroblastoma cell line SH-SY5Y. Following differentiation, targeting the dopaminergic cells with a dopaminergic neurotoxin such as 6-hydroxydopamine (6-OHDA) results in increased expression and aggregation of α-Synuclein, alongside mitochondrial dysfunction, oxidative stress, and neuronal cell death (Ioghen et al., 2023). Thus, this model serves as a robust in vitro phenotypic mimic of PD.
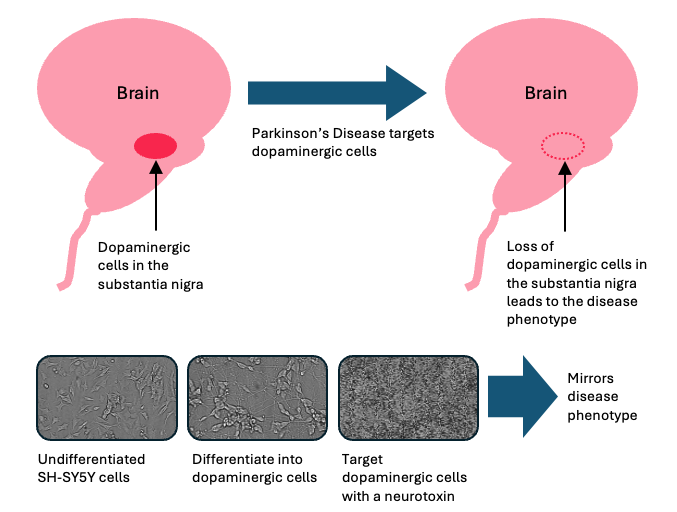
Figure 1: Relationship of the Parkinson’s Disease process leading to disease phenotype and the requirement for differentiating SH-SY5Y cells.
Differentiation is achieved through the sequential incubation of SH-SY5Y cells with retinoic acid for 5 days, followed by brain-derived neurotrophic factor (BDNF) for a further 5 days (Dravid et al., 2021). Gradual changes in cellular morphology can be observed during this period (Figure 2). The differentiated dopaminergic phenotype is validated by an increase in dopamine receptor D2 (DRD2) gene expression by qPCR, alongside increased expression of α-Synuclein by in-cell ELISA. DRD2 and α-Synuclein have been implicated in PD and neuroinflammation. Polymorphisms of the DRD2 gene have been linked to increased risk of developing PD, whilst the accumulation of abnormal forms of a-Synuclein are believed to perturb dopaminergic signalling (Magistrelli et al., 2021; Calabresi et al., 2023).
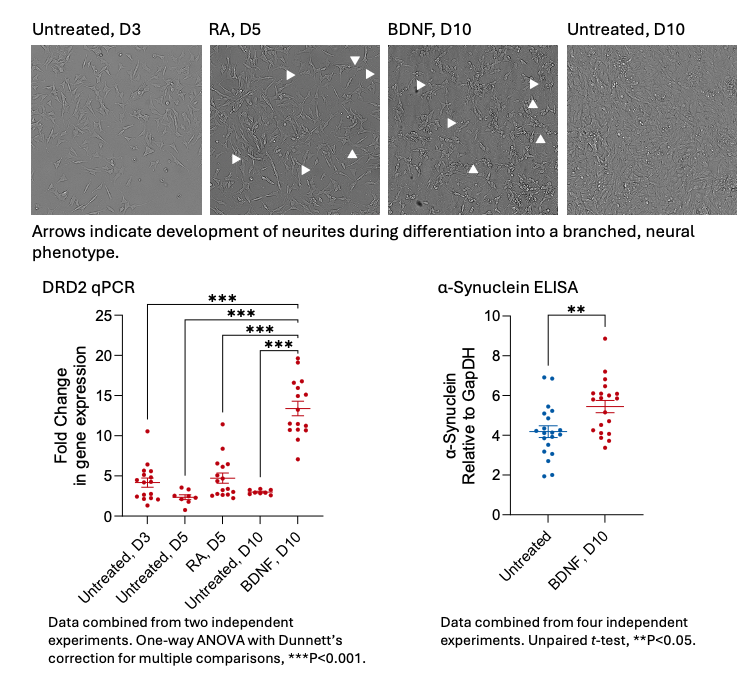

Figure 2: Differentiation of SH-SY5Y cells into dopaminergic cells can be stimulated by sequential treatment with Retinoic Acid (RA) for 5 days and then brain-derived neurotrophic factor (BDNF) for a further 5 days. This leads to changes in morphology, noticeably the development and extension of a network of neurites (indicated by arrowheads on the micrographs, along with raised expression levels of the dopamine D2 receptor (DRD2, by means of qPCR) and α-Synuclein (by means of an in-cell ELISA).
Whilst differentiation can be visualised by live cell imaging on a JuliStage, it cannot alone reflect a true PD phenotype, which involves the death of dopaminergic cells. To this end, differentiated cells were treated with 6-OHDA, which has been shown to induce the development of PD-like symptoms and the death of dopaminergic neurons in in vivo models (Guimarães et al., 2021). By way of microscopic examination, we found that 24 h treatment with 6-OHDA induced a concentration-dependent increase in cell death in differentiated cells at lower concentrations than in undifferentiated populations (quantified by measuring cell confluency). This increased sensitivity to 6-OHDA was attributed to the differentiation to a dopaminergic phenotype, confirmed by a ~13-fold increase in DRD2 gene expression via qPCR (Figure 3). In agreement with the cell confluency data, assessment of cell death using an LDH assay revealed a greater response to 6-OHDA in differentiated cells when compared to undifferentiated cells. Furthermore, this was associated with increased levels of reactive oxygen species in the differentiated cells (labelled with H2-DCFDA).
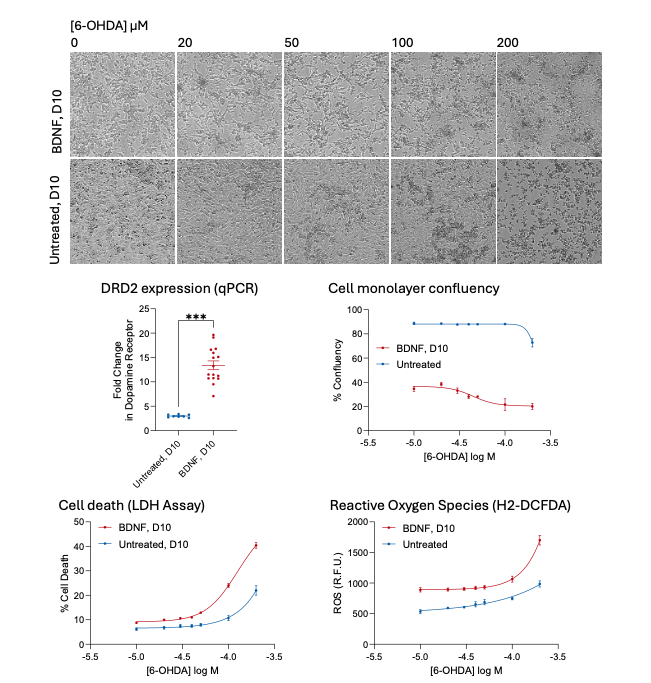

Figure 3: Micrographs demonstrate that differentiated SH-SY5Y cells (D10, treated with BDNF) are more sensitive to increasing the concentration of 6-Hydroxydopamine (6-OHDA) than undifferentiated cells (D10, untreated) and begin to die off. This accompanies the increased expression of the dopamine 2 receptor DRD2 and can be quantified by a loss of cell confluency which differs between differentiated and undifferentiated cells. Furthermore, cell death (LDH assay) and measurement of intracellular ROS (H2-DCFDA staining) exhibit differences in the sensitivity between differentiated and undifferentiated cells.
An in-cell ELISA was used to determine whether 6-OHDA could modify the expression of a-Synuclein; this identified a gradual concentration-dependent loss in undifferentiated cells (Figure 4). In our hands, low concentrations of 6-OHDA induced an increase in α-Synuclein expression in differentiated cells which was comparable to published results (Ioghen et al., 2023). However, this was reversed as the concentration of 6-OHDA was increased, leading to a loss of α-Synuclein expression. Whilst many published studies use western blotting to assess α-Synuclein expression and phosphorylation, our study used in-cell ELISA technology, which may identify alternative trends. The primary difference between in-cell ELISA and traditional western blotting is a lack of control over sample input levels. In-cell ELISAs are susceptible to changes in cell numbers if treatments have an adverse effect on cell viability; this is compensated for in traditional western blots by normalisation of input protein levels following a BCA protein assay.


Figure 4: Differentiated SH-SY5Y cells express higher levels of α-Synuclein than undifferentiated cells (quantified by means of an in-cell ELISA assay-Figure 2). This is modified by incubation with 6-hydroxydopamine (6-OHDA); differentiated cells (BDNF, D10) expressing higher levels of the dopamine receptor DRD2 (Figure 2) show an increase in expression of α-Synuclein at lower concentrations of 6-OHDA, which drops off and falls below that of untreated cells (untreated, D10) at higher, cytotoxic concentrations (greater than 50 μM 6-OHDA). Data represents the mean of two independent experiments. Horizontal lines represent mean of unstimulated cells (no 6-OHDA)±S.E.M.
The plant-derived compound Salidroside possesses potential neuroprotective activity, making it a suitable reference compound for attempts to reduce/reverse the PD-associated phenotype in vitro (Chen et al., 2019; Li et al., 2018). In our model, we successfully demonstrated that 24 h pre-treatment with Salidroside followed by 24 h stimulation with 6-OHDA resulted in a reduction in cell death and partial inhibition of changes in α-Synuclein expression by way of an LDH assay and in-cell ELISA, respectively (Figure 5).
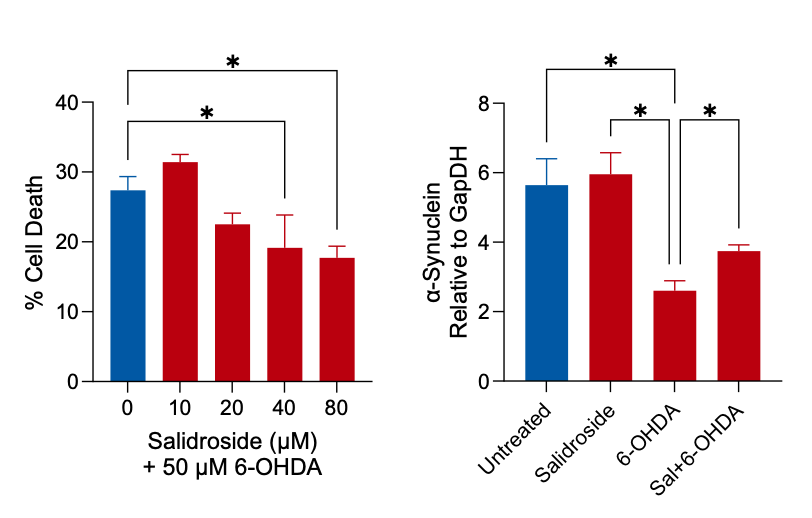

Figure 5: Cultures of differentiated SH-SY5Y cells were incubated with the potential neuroprotective compound Salidroside for 24 h, prior to incubation with 50 µM 6-OHDA for a further 24 h. Cell death induced by 6-OHDA was reduced following pre-treatment with Salidroside (LDH assay, red columns) compared to cells treated with 6-OHDA only (blue column). Cells were exposed to the same treatment regime, with the exception that the hydroxydopamine concentration employed was 100 µM, and an in-cell ELISA assay demonstrated that 100 μM 6-OHDA induced a significant reduction in the expression of α-Synuclein. This was significantly altered following pre-incubation of cells with 80 μM Salidroside.
Alzheimer’s Disease
Alzheimer’s Disease is already the most common form of dementia and globally, cases continue to increase significantly (Bhole et al., 2023). Neuroinflammation has been implicated in the development of the disease phenotype (Giri et al., 2024) and is a risk factor contributing to disease onset, which is associated with the accumulation of the amyloid-b plaques classically associated with AD (Jain et al., 2024).
Amyloid-β is derived from the amyloid precursor protein (APP), which is natively expressed in undifferentiated SH-SY5Y cells. During amyloidogenic processing, the APP is cleaved by BACE (b-site amyloid precursor protein cleaving enzyme) which releases the soluble sAPPβ fragment from the cell. This leaves the C99 transmembrane β-C-terminal fragment which is subsequently cleaved by the g-secretase, releasing amyloidβ42 from the cell (Liu et al., 2021). The g-secretase-mediated cleavage requires the BACE-mediated cleavage to occur first. Shed amyloidβ42 proteins can oligomerize, forming the amyloid plaques associated with AD. Microglial cells in the brain can detect amyloidβ42, leading to a neuroinflammatory response. In the healthy brain, microglia take up amyloidβ42 by phagocytosis, but this may be deficient in AD (Thomas et al., 2022).
Stimulation of cells with LPS activates amyloidogenic processing of APP leading to a loss of cytoplasmic APP, which can be detected using a commercially available ELISA (Figure 6). This is accompanied by increased levels of sAPPb and amyloidb42 in the media. Little change is seen in the levels of the C99 b-C-terminal fragment. Thus, the SH-SY5Y cell line provides a platform for studying inflammation and processing of amyloidβ42 in the context of modelling AD in the laboratory; this can be used in high-throughput screening of test compounds.


Figure 6: Schematic of an ELISA-based pipeline for the assessment of the amyloidogenic processing of the intracellular amyloid precursor protein APP, leading to shedding of amyloidβ42 into the cell culture media following stimulation with LPS to mimic neuroinflammatory conditions.
Amyloidogenic processing is a multi-step pathway, and each step can be targeted by pharmacological inhibitors (Figure 7). The role of BACE can be determined by incubating cells with Verubecestat or LY2886721. This prevents the LPS-induced reduction of intracellular APP and inhibits the shedding of sAPPb and amyloidb42 into the media, confirming the role of BACE in this pathway. Verubecestat has been included in recent clinical trials as a potential therapeutic intervention designed to delay AD progression (Voss et al., 2023). The inhibition of the g-secretase with DAPT results in the enrichment of the C99 b-C-terminal fragment: by preventing this step, the β-C-terminal fragment cannot undergo further processing and so detectable levels increase.
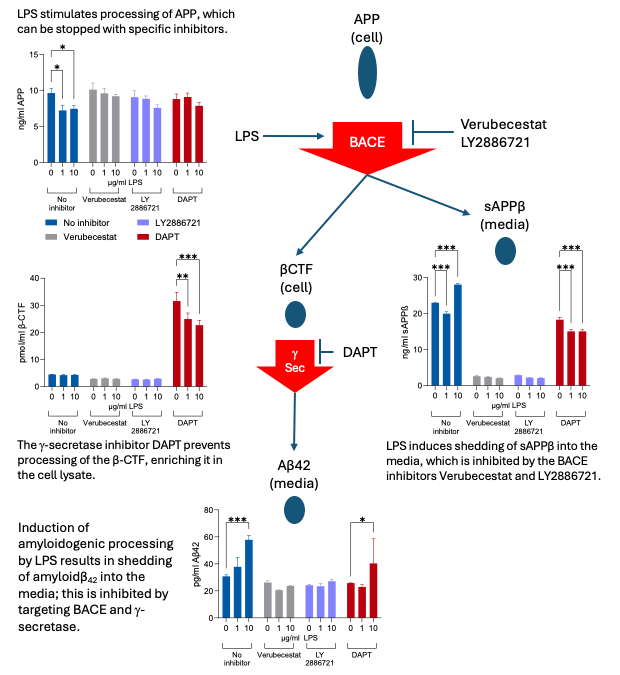

Figure 7: Pharmacological intervention results in inhibition of amyloidogenic processing of APP. Cells were incubated with the BACE inhibitors Verubecestat and LY2886721 or the g-secretase inhibitor DAPT prior to inducing neuroinflammatory conditions with LPS. Proteins at each stage of the pathway (iAPP, βCTF, sAPPβ, amyloidβ42) were analysed by ELISA and changes correlated with where the pathway was blocked. Each protein assessed in an aliquot of the same sample, so levels were directly related.
Alternatively, the amyloid precursor protein can be processed through the non-amyloidogenic pathway. The major sheddase is ADAM10 (Elsworthy et al., 2022), and this pathway prevents the release of amyloidβ42. ADAM10 function can be stimulated with Ionomycin and blocked with the metalloproteinase inhibitor GM6001. Treatment of SH-SY5Y cells with Ionomycin resulted in a loss of intracellular APP (Figure 8), which was inhibited by GM6001 but not by DAPT. Ionomycin did not induce the shedding of amyloidβ42 into the media, indicating that the activation of the non-amyloidogenic pathway rather than the amyloidogenic pathway resulted in the reduction of detectable cellular APP.
This data demonstrates that we can identify processing pathways using off-the-shelf enzymatic inhibitors and ELISAs for known biomarkers; this forms a control for the assessment of potential novel pharmacological interventions during drug development and screening.
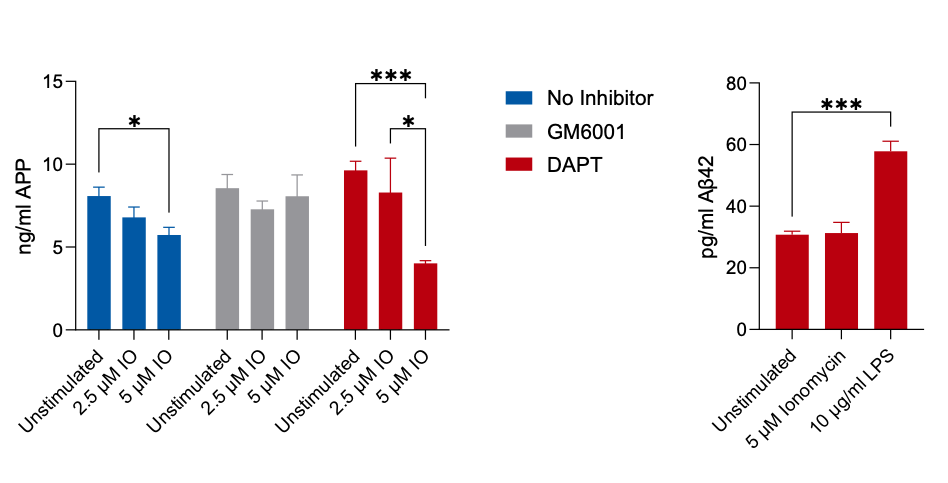

Figure 8: Stimulation of non-amyloidogenic processing of the amyloid precursor protein with Ionomycin results in a concentration-dependent reduction in APP. This is prevented by the metalloproteinase inhibitor GM6001 but not by the g-secretase inhibitor DAPT. Unlike LPS stimulation, Ionomycin does not induce an increase in amyloidβ42 into the culture media.
Three-dimensional spheroid models
The above models are all based on simple two-dimensional culture systems, where cells are grown as a monolayer on a plastic surface. To progress from these artificial environments, Cellomatics Biosciences has developed alternative spheroid models with greater biological relevance. Here, cells grow naturally in a 3D environment, leading us to wonder if the functionality of SH-SY5Y spheroids would differ to the traditional 2D models. Importantly, could single spheroids find practical application in high-throughput screening (Figure 9), or would we require multiple spheroids bulked together to achieve a detectable readout?
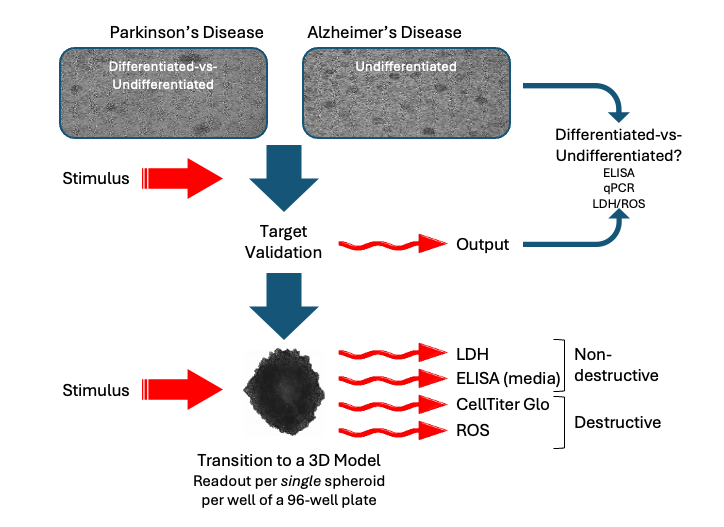

Figure 9: Schematic of experimental design for high throughput analyses using SH-SY5Y cells, from validation of targets in classical 2D culture prior to treatment of spheroids, where multiple endpoints can be assessed in a single spheroid.
To address these questions, we generated stable spheroids from SH-SY5Y cells and induced cell death by treating them with 6-OHDA (Figure 10). The cells forming the spheroid were killed in a concentration-dependent manner by 6-OHDA (identified using an LDH assay). Furthermore, we were able to demonstrate that 6-OHDA treatment raised the levels of reactive oxygen species within a single spheroid labelled with H2-DCFDA. Combined, this data provides the basis for transferring the PD model into a 3D format.


Figure 10: Example data from SH-SY5Y spheroids. Exposure to 6-OHDA alters spheroid morphology and induces a concentration-dependent increase LDH activity in media (expressed as % of untreated cells); intracellular staining with H2-DCFDA demonstrates that 6-OHDA induces an increase in reactive oxygen species. Induction of neuroinflammatory conditions in individual spheroids (n=4) with LPS induces a significant increase in amyloidβ42 in cell culture media; this is inhibited by Verubecestat, confirming stimulation of the amyloidogenic pathway. In comparison, an alternative BACE inhibitor, LY2886721, does not significantly inhibit the pathway. Stimulation of non-amyloidogenic processing with Ionomycin induces a small but non-significant increase in shed amyloidβ42 in the media, which is inhibited by the metalloproteinase inhibitor GM6001.
Clearly, we can use biochemical markers to analyse spheroid phenotype. However, can we look at more specific markers of neuroinflammation in a single spheroid? To answer this, SH-SY5Y spheroids were treated with the enzyme inhibitors GM6001, Verubecestat, LY2886721 or DAPT. Non-amyloidogenic or amyloidogenic processing of APP was stimulated with Ionomycin or LPS, respectively. Conditioned media from a single spheroid in each well of a 96-well plate was analysed using a commercially available ELISA kit (Figure 10). This was sufficiently sensitive to measure the stimulation of release of amyloidβ42 from a single spheroid; this release was inhibited by Verubecestat, but not by LY2886721. This demonstrated that (i) we can analyse a single spheroid by means of an ELISA, and that (ii) we can run plate-based assays on spheroids cultured in a 96-well plate as a high-throughput model, using a suitable ELISA or biochemical stain as the endpoint.
Summary
The frequency of dementia cases is increasing with every published report and each health-related news broadcast. This only emphasises the constant need to drive forward novel improved methods for testing potential treatments or biomarkers. Here, we have taken our existing platforms and demonstrated their effectiveness in studying the disease phenotypes associated with different forms of dementia. Echoing the ability of the human brain to multi-task, we can interrogate a single well of cells, or even a single spheroid, with simultaneous queries to generate correlative data from multiplexed outputs, in a high-throughput system.







