In this blog, Dr Oana Blair, Bioassay Research Scientist at Cellomatics, discusses osteoarthritis which is the most common type of arthritis in the UK, and explains how Cellomatics has developed bespoke and customised assays to support clients working in this therapeutic area.
Osteoarthritis (OA) or arthrosis is a highly predominant rheumatic degenerative condition that causes the cartilage protecting the end of your bones to wear down resulting in pain, swelling and mobility problems. Data from Global Burden of Diseases, Injuries and Risk Factors Study 2017 (GBD 2017) revealed that this disorder affected more than 300 million people globally.
According to the NHS, OA is the most common type of arthritis in the UK. There is no direct cause of OA and anyone could be diagnosed with this condition. However, this condition is most likely to affect those where any of the following categories apply: women over 40 years old, genetic predisposition (if family members had the same condition), weight, prior joint injuries, or pre-existing conditions that already have an impact on the joints such as gout or rheumatoid arthritis.
Clinically, the knee is one of the most common areas of OA. Recently, a study that has used data from the UK general practices has revealed that the incidence of OA in the knee and hand is far greater in women between the ages of 50 years and 75 years compared to men (Morgan et al, 2019).
OA can be triggered by several underlying factors including mechanical, inflammatory, and metabolic which ultimately result in destruction and failure of the synovial joint.
Under normal, physiological conditions, the cartilage composition (Type II collagen, proteoglycans, and chondrocytes) is maintained by the balanced activity of the degenerative and synthetic enzymes.
During the OA process, the cartilage structure is modified, and the destructive enzymes are un-regulated, leading to degradation and loss of collagen and proteoglycans from the matrix. Furthermore, in response to this imbalance, chondrocytes are triggered to proliferate and replace the proteoglycan and collagen molecules. However, the repair process is ineffective, resulting in an increase in cartilage matrix degeneration.
The principal enzymes involved in cartilage degeneration are the matrix metalloproteinases (MMPs). These enzymes are produced by both the chondrocytes and synovial cells and in normal physiological conditions, the levels of these enzymes are strictly controlled by several MMPs inhibitors. Conversely, in OA the MMPs production is significantly increased, and the existing inhibitors are outmatched, resulting in chondrocyte hypertrophy and cell death.
For many years, various therapeutic approaches have been focused on pain management and quality of life in OA patients. Recently, novel anti-inflammatory therapeutic approaches are currently being developed and pre-clinically screened. In recent years, various OA in vitro models have been developed or redefined. Establishing accurate in vitro models plays a critical role when developing OA therapies. Several 2D monolayer cellular models have been developed including primary synoviocytes and chondrocytes extracted from human tissue. The main advantages of using human primary cells for screening include the high throughput, low cost, and suitability to evaluate cellular differences in phenotype studies. Furthermore, the 2D cell models like chondrocytes and synoviocytes that respond to cytokine stimulation constitute an ideal screening model for the anti-inflammatory drug molecules which could be quantified by measuring the levels of inflammatory mediators such as IL-6, MMP-1, MMP-3, TNF-a.
These are well-established and validated markers that have extensively been used in pre-clinical screening. In addition, various drug molecules targeting the pro-inflammatory cytokines have been developed are currently in clinical trials. The review by Cai et al. 2021 highlights the latest emerging pharmacological therapies developed to reduce structural damage (Table 1, Cai et al. 2021).
At Cellomatics, we utilised our bespoke assay development skills to develop 2D multicellular models of OA that can support client pre-clinical in vitro screening. Additionally, we have in-house expertise in culturing and maintaining primary chondrocytes obtained from healthy and OA donors. Our capabilities include the assessment of hypertrophic markers, extracellular matrix deposition, cartilage degradation and inflammation using multiplex immunoassays which measure the levels of markers associated with OA.
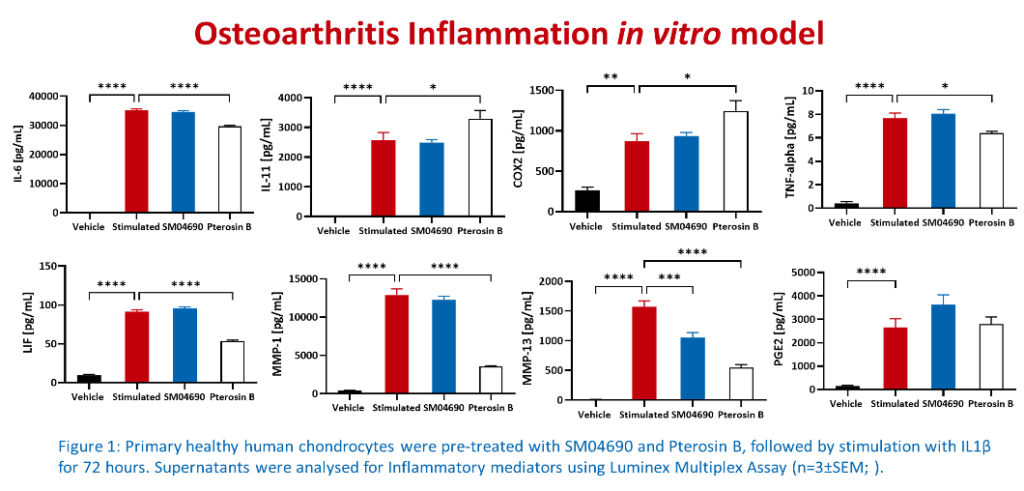
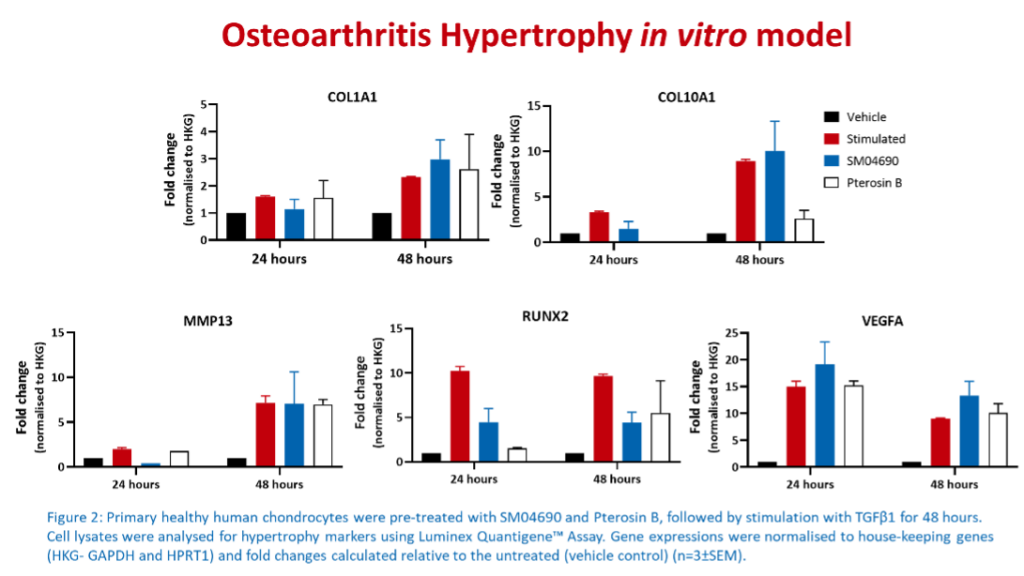

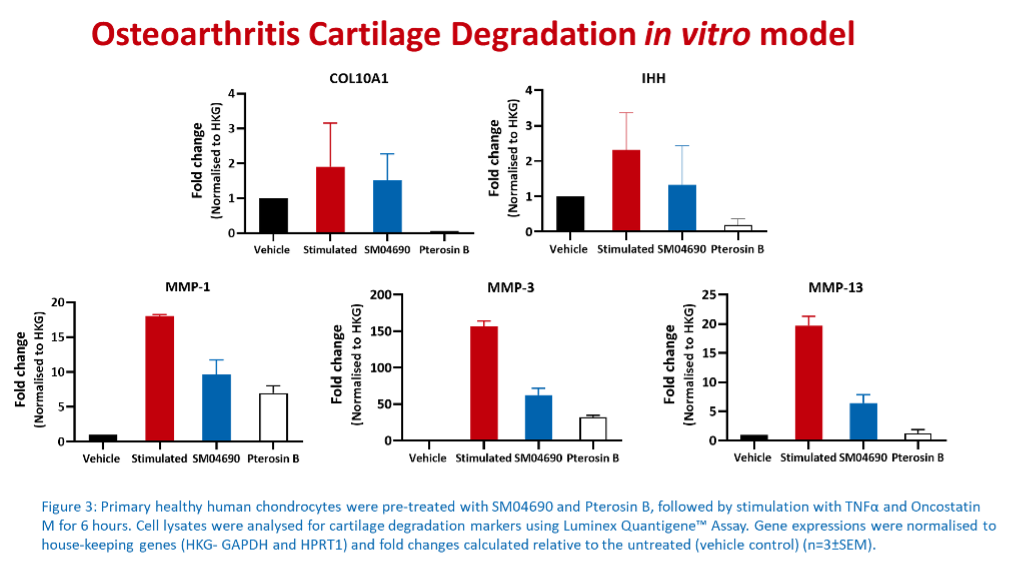

Multiplex immunoassays are the preferred methods used for pre-clinical in vitro screening because they can simultaneously detect and quantify multiple analytes in a single sample, have high sensitivity, reproducibility, and high throughput capacity. Furthermore, these assays are very cost-effective and time-efficient, allowing the researcher to select the most promising therapeutic agents to be passed onto the next testing stages.
If you would like to discuss the in vitro models developed for OA, or our capabilities in supporting drug discovery screening assays, please do not hesitate to get in touch with us.
References:
GBD 2017: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)32279-7/fulltext
Morgan et al 2019: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6858070/
Cai et al 2021: https://www.frontiersin.org/articles/10.3389/fphar.2021.645842/full










