Immuno-oncology Assays
Antibody Dependent Cellular Cytotoxicity (ADCC)
Antibody-dependent cellular cytotoxicity (ADCC), also referred to as antibody-dependent cell-mediated cytotoxicity, is a mechanism of cell-mediated immune defence whereby an effector cell of the immune system actively lyses a target cell, whose membrane-surface antigens have been bound by specific antibodies. It is one of the mechanisms through which antibodies, as part of the humoral immune response, can act to limit and contain infection.
- Examine ADCC activity of your proprietary therapeutic antibody using physiologically relevant in vitro model
- Bespoke ADCC assays using target and effector cells (PBMCs, T-cells, NK cells) of your choice
- Develop cell lines expressing target antigen of your interest
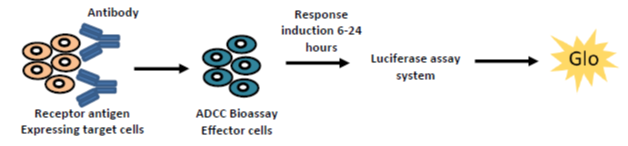
1. Transfecting target cells with GFP and measuring the loss of intracellular GFP following incubation with effector cells and therapeutic test antibody as a surrogate for cytotoxicity. This model provides you the flexibility to work with different combinations of target/effector cells and test antibodies and can be applied for validation projects
Antibody-dependent cellular cytotoxicity (ADCC)
Therapeutic antibodies often mediate direct killing of cancer cells through antibody dependent cell mediated cytotoxicity (ADCC). This can be detected using the luciferase assay system that measures NFAT (nuclear factor of activated T-cells) mediated cell death via an increase in luminescence.
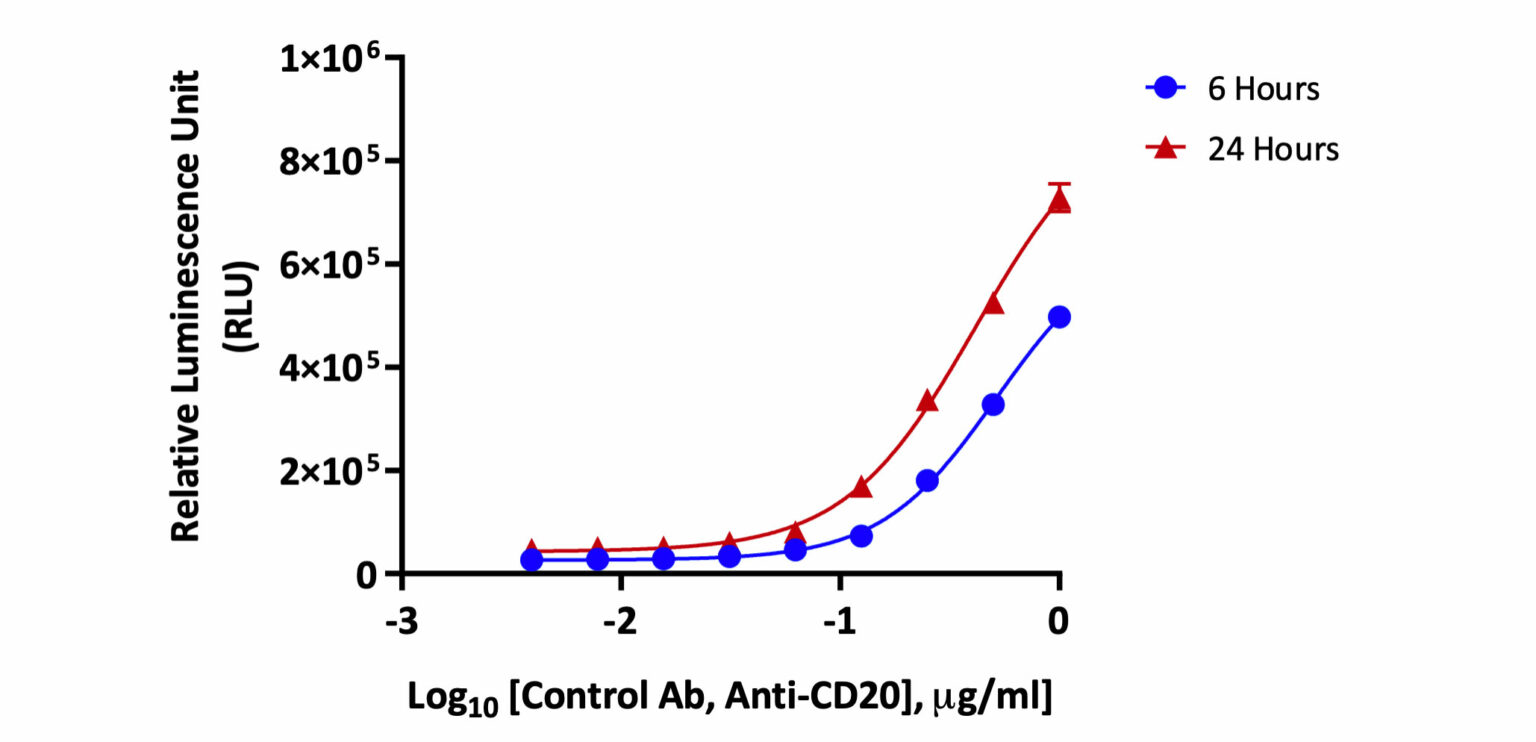
Target Raji cells were treated with increasing concentrations of anti-CD20 antibody and co-cultured with Jurkat effector cells expressing FcγRIIIa for 6 hours or 24 hours. Antibody binding to antigens on the target cell surface and subsequent interaction of the Fc portion with FcγRIIIa receptors on the effector cell leads to activation of NFAT (nuclear factor of activated T-cells) pathway and cell death. Relative Luminescence Units (RLU) corresponding to an increase in the target cell death was measured and recorded.
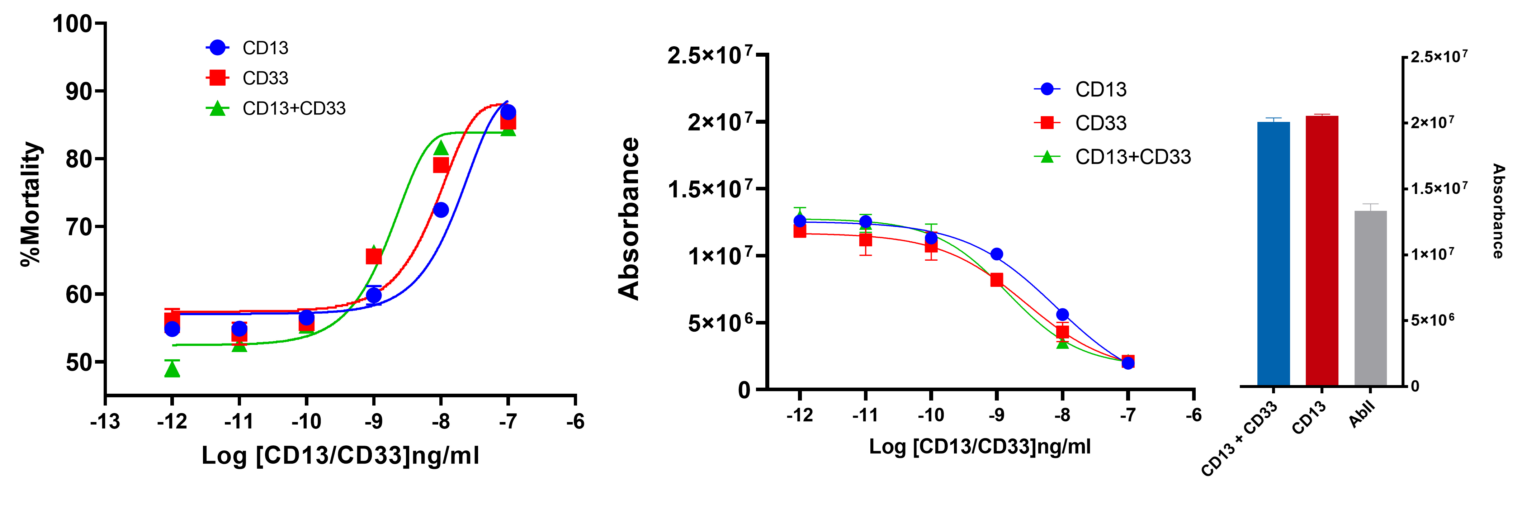
ADC assay was performed using Human Mast Cells 1.2 (HMC1.2). CD13 and CD33, respective markers for solid tumours and acute myeloid leukaemia, are targeted using secondary antibody MMAE-conjugated. CD13+ and CD33+ HMC1.2 cells were exposed to primary antibodies CD13 and CD33 specific, followed by increasing concentrations of secondary antibody MMAE-conjugated (0 ng/ml to 100 ng/ml). Cytotoxicity was assessed by ATP release assay. Percentage of mortality was calculated relative to cells treated with primary antibodies only. Each point is the average of 4 replicates. Bars represent the Standard Error Mean (SEM).
Assessment of ADCC via GFP release
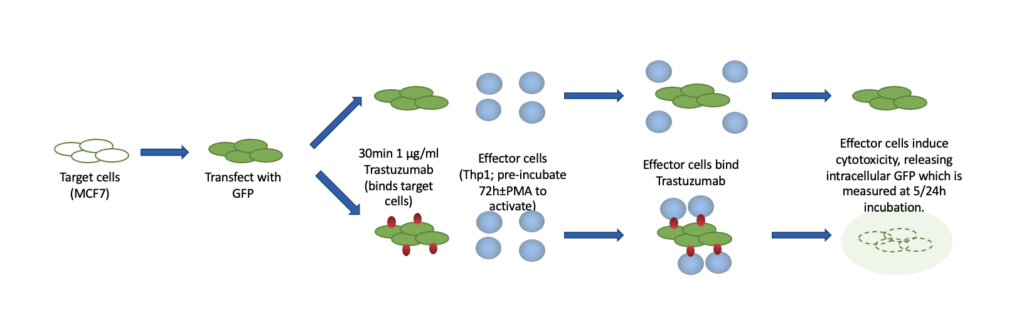
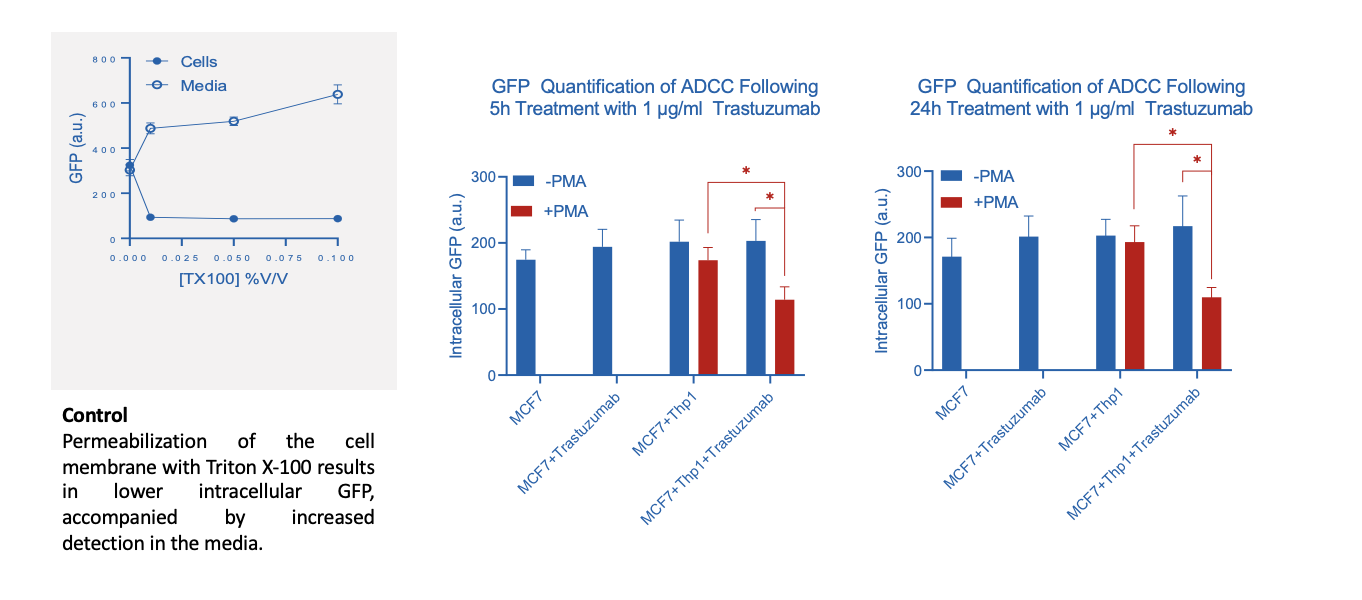
Incubation of GFP labelled MCF7 (target) cells with both Trastuzumab and PMA activated Thp1 (effector) cells induces a loss of intracellular GFP suggesting an increased antibody-dependent cytotoxic effect on MCF7
Request a consultation with Cellomatics Biosciences today
Our experienced team of in vitro laboratory scientists will work with you to understand your project and provide a bespoke project plan with a professional, flexible service and a fast turnaround time.
To request a consultation where we can discuss your exact requirements, please contact Cellomatics Biosciences.