
In our first instalment of Science Communication our senior bioassay scientist delves into immune cell chemotaxis and the capabilities we have for this research at Cellomatics.
In the intricate theatre of the human immune system, a meticulously orchestrated performance unfolds, choreographed by a specialised ensemble of immune cells. These extraordinary cellular entities exhibit a unique capacity to traverse the intricate terrain of the human body, analogous to microscopic investigators adept at identifying and responding to potential threats. This sophisticated dance, scientifically termed chemotaxis, is a critical phenomenon in the body’s defence against infections, injuries, and diseases. In this exploration, we delve into the intricacies of immune cell chemotaxis, elucidating its mechanisms, significance, and the delicate equilibrium between amplification and therapeutic targeting, while acknowledging the potential antagonism of chemotaxis.
At its essence, chemotaxis denotes the directed movement of cells along a chemical gradient—a pivotal process orchestrating the timely arrival of immune cells at sites of infection or injury. Immune cells, acting as vigilant sentinels, continually patrol the body, attuned to signals indicative of potential threats.
Chemotaxis constitutes a meticulously regulated orchestration involving diverse cell types, signalling molecules, and receptors. During an infection, damaged cells release chemokines, functioning as molecular beacons guiding immune cells to the locus of disturbance. Equipped with receptors finely tuned to these chemokines, immune cells perceive the chemical gradient and migrate with precision toward the site of infection, engendering a dynamic and awe-inspiring response.
The journey of immune cells across the body is not devoid of challenges. Tissues and organs present intricate terrains, necessitating immune cells to navigate through complex landscapes to reach their destinations. This intricate process encompasses coordinated steps, including chemokine gradient sensing, the extension of cellular protrusions known as pseudopodia, and directed migration toward the source of chemical signals.
Central to chemotaxis is its dynamic nature. Immune cells continually adapt their direction and speed in response to evolving chemical cues, a crucial attribute for an effective immune response, ensuring cells reach their intended targets promptly.
Chemotaxis functions as the immune system’s navigational system, steering immune cells to the front lines where their presence is urgently required. Crucial to the body’s defence against infections, it plays a pivotal role in the process of wound healing. An efficiently operating chemotactic system ensures that the appropriate immune cells are recruited to precise locations at optimal times.
In instances of bacterial invasion, specific chemokines released by infected tissues attract neutrophils, the foot soldiers of the immune system. Guided by the chemokine gradient, neutrophils converge at the infection site, forming a formidable defence against invading pathogens. The precision of chemotaxis not only augments the immune response but also mitigates collateral damage to healthy tissues.
The notion of enhancing chemotaxis holds promise in fortifying the immune response, yet it presents challenges and potential risks. Researchers are actively exploring strategies to harness this process for the improvement of immune cell recruitment and function, especially in cases where the natural response proves insufficient.
One avenue of investigation involves the development of therapeutic strategies to selectively enhance chemotaxis. This may encompass the engineering of immune cells to express elevated levels of specific chemokine receptors or the administration of synthetic chemokines to amplify the signalling gradient. The objective is to achieve a more robust and precisely targeted immune response, particularly in individuals with compromised immune systems or chronic conditions.
However, the augmentation of chemotaxis necessitates cautious consideration. Unbridled chemotaxis can lead to hyperactive immune responses, culminating in chronic inflammation and autoimmune disorders. Striking the right balance is imperative to circumvent unintended consequences, highlighting the importance of a measured and judicious approach to manipulating immune cell chemotaxis for therapeutic purposes.
While chemotaxis is essential for the effective mobilisation of immune cells to sites of infection or injury, harnessing its inhibition has emerged as a compelling strategy for treating various diseases. This approach involves modulating the precision and intensity of immune cell migration, presenting a novel frontier in therapeutic intervention.
Many diseases, ranging from chronic inflammatory conditions to certain cancers, involve dysregulated immune responses characterised by excessive or misguided chemotaxis. Inflammatory disorders often exhibit uncontrolled immune cell migration, leading to sustained inflammation and tissue damage. Similarly, cancer cells exploit chemotactic mechanisms to invade surrounding tissues and metastasize to distant sites. Inhibition of chemotaxis, therefore, presents a promising avenue for mitigating the progression of such diseases.
Autoimmune disorders arise when the immune system mistakenly attacks healthy tissues, leading to chronic inflammation and tissue damage. In diseases like rheumatoid arthritis and multiple sclerosis, aberrant chemotaxis contributes to the recruitment of immune cells to healthy tissues, exacerbating the autoimmune response. Inhibiting chemotaxis offers a strategic approach to attenuate the influx of immune cells and temper the destructive immune response, potentially providing relief for individuals suffering from autoimmune conditions.
Cancer metastasis, the spread of cancer cells to distant organs, is a critical factor in the progression of malignancies. Chemotaxis facilitates the migration of cancer cells towards specific microenvironments that support their survival and growth. By inhibiting chemotaxis, researchers aim to disrupt this migratory process, impeding the ability of cancer cells to metastasize. This approach holds promise as an adjunct therapy to conventional cancer treatments, potentially improving outcomes and reducing the likelihood of disease recurrence.
In the context of infectious diseases, modulation of chemotaxis offers a multifaceted strategy. In some cases, hyperactive chemotaxis contributes to excessive inflammation, tissue damage, and immunopathology. Inhibiting chemotaxis can help dampen the exaggerated immune response, promoting a more balanced and controlled reaction to the invading pathogen. Conversely, in certain infections, enhancing chemotaxis may be beneficial to bolster the immune response. Striking the right balance between inhibition and enhancement is crucial in tailoring therapeutic strategies to the specific characteristics of each infectious agent.
At Cellomatics, we utilised our immunological skills to support clients in pre-clinical compound screening. By leveraging our ability to access freshly isolated immune cells onsite, we irradicate any issues arising from long term storage of blood. Our capabilities include measuring both end-point luminescence and fluorescence-based techniques, allowing us to measure significant numbers of compounds in a single experiment, generating multi-point concentration response curves in a single sitting.
Primary human Neutrophil Chemotaxis
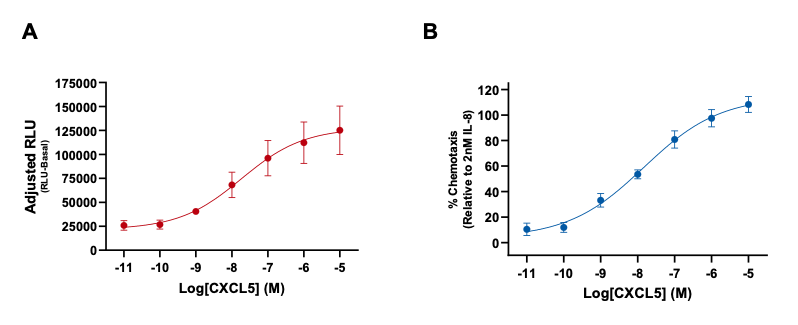
Figure 1: Measurement of primary neutrophil chemotaxis by the chemoattractant CXCL5. (A) CXCL5-induced chemotaxis as adjusted RLU (RLU-basal) values and (B) percentage increase of neutrophil chemotaxis compared to 2nM IL-8. Data are represented as n=3 ± SEM.
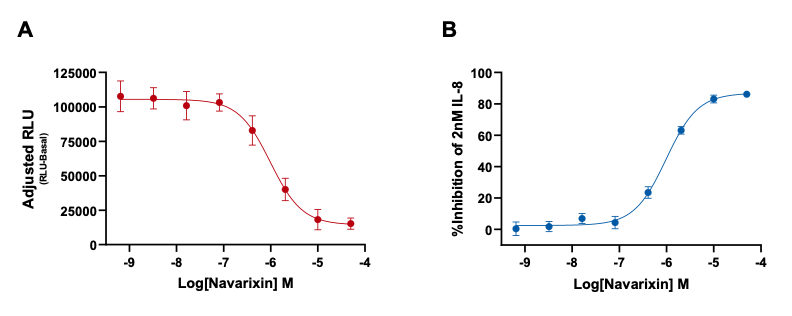
Figure 2: The inhibition of IL-8 (CXCL8) induced chemotaxis by the selective CXCR1/2 antagonist Navarixin. (A) Navarixin inhibition of IL-8 induced chemotaxis as adjusted RLU (RLU-basal) values and (B) percentage inhibition of IL-8 induced neutrophil chemotaxis by Navarixin. Data are represented as n=3 ± SEM.
Primary human Monocyte Chemotaxis
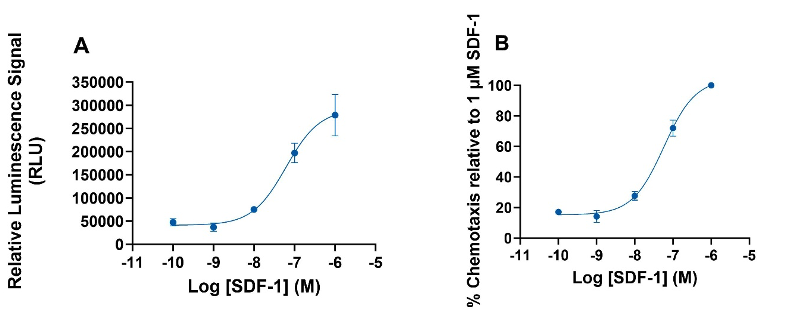
Figure 3: Chemotaxis of freshly isolated CD14+ monocytes induced by SDF1-α. (A) Luminescence signal (with basal luminescence removed) produced by SDF1-α over a range of concentrations. (B) Concentration-response curve produced as percentage chemotaxis relative to 1µM SDF-1α. The results are the mean ± SEM of 3 donors.
To learn more about our immunology assays, visit our dedicated page here.










