INNOBEX™ In vitro model of the blood-brain barrier
The blood-brain barrier (BBB) is an important and highly selective structure in the brain, responsible for the transport of nutrients while preventing hazardous substances or pathogens entering from the bloodstream to the brain. In vitro models of the BBB that accurately represent the complex physiology of the BBB in vivo are highly sought after, given its role in health and disease as well as the challenges it imposes on drug discovery projects targeting the CNS. BBB dysfunction is a hallmark of numerous diseases of the CNS, including neurodegenerative diseases (Alzheimer’s and Parkinson’s disease), and ischaemic stroke. However, mechanisms associated with other actions of the BBB in health and disease can also be studied, such as drug permeability.
Many companies have developed various in vitro blood-brain barrier (BBB) models, ranging from simple monoculture systems to more advanced organ-on-a-chip platforms. While these models offer valuable insights, each has its limitations. To address the diverse challenges posed by existing in vitro BBB models, our solution was to develop a high-throughput, customizable tri-culture model that better preserves the complex physiology of the BBB. This innovative approach aims to overcome the current gaps in BBB research and provide a more reliable and versatile solution.
Our trademark model, INNOBEX™, consists of primary human brain microvascular endothelial cells (HBMVECs), astrocytes, and pericytes in a 3D transwell format to simulate the separation between the blood (apical chamber) and the brain (basal chamber) whilst maintaining cell-to-cell interactions and forming stable barrier properties. Our INNOBEX™ model has been validated for the expression of tight junction proteins, common transporter targets, and by using trans-endothelial electrical resistance (TEER) and FITC-Dextran we have demonstrated that barrier integrity can be manipulated in response to different stimuli.
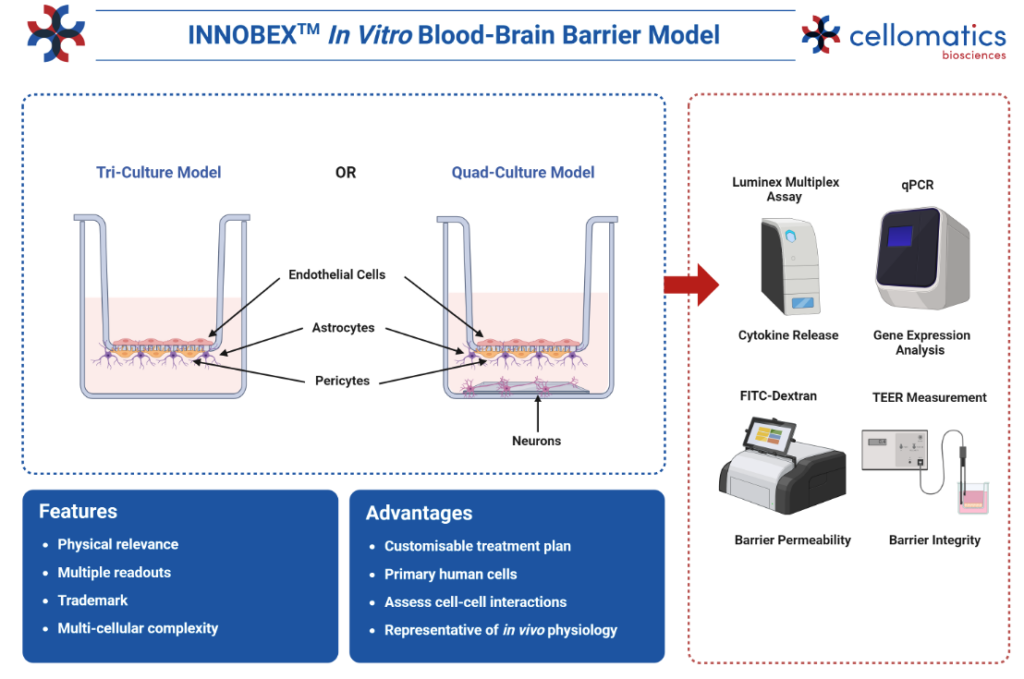
Using our INNOBEX™ model of the blood-brain barrier, you can explore a range of applications, including:
- The permeability of novel compounds in your drug discovery pipelines via LC-MS/MS analysis1
- Explore the mechanisms associated with neuroinflammation in neurodegenerative diseases, e.g., Alzheimer’s2-3 and Parkinson’s disease4
- Mechanisms associated with immune cell migration across the BBB5-6
- Test the efficacy of drugs targeting cancer metastasis across the BBB7
Cell-to-cell interactions
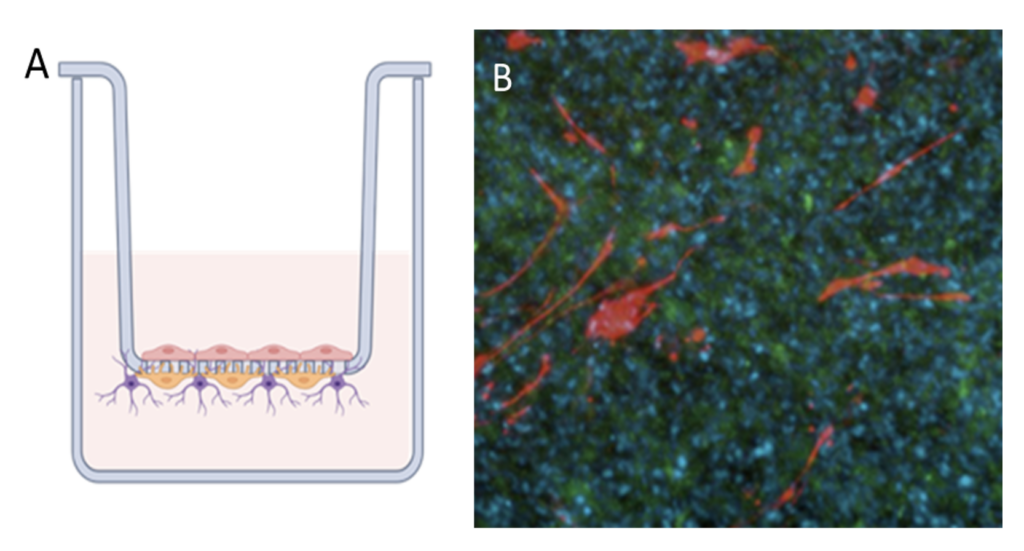
The blood brain barrier model is grown in transwells (A). Astrocytes and pericytes are seeded on the basal side of the membrane and HBMVECs on the apical side, growth and monolayer formation is monitored. Immunofluorescence demonstrating the different cell types (B). Astrocytes are detected by GFAP (red), and HBMVEC by Smooth muscle actin (green). DAPI (blue) is used to detect nuclei of all cell types.
Expression of BBB transporters
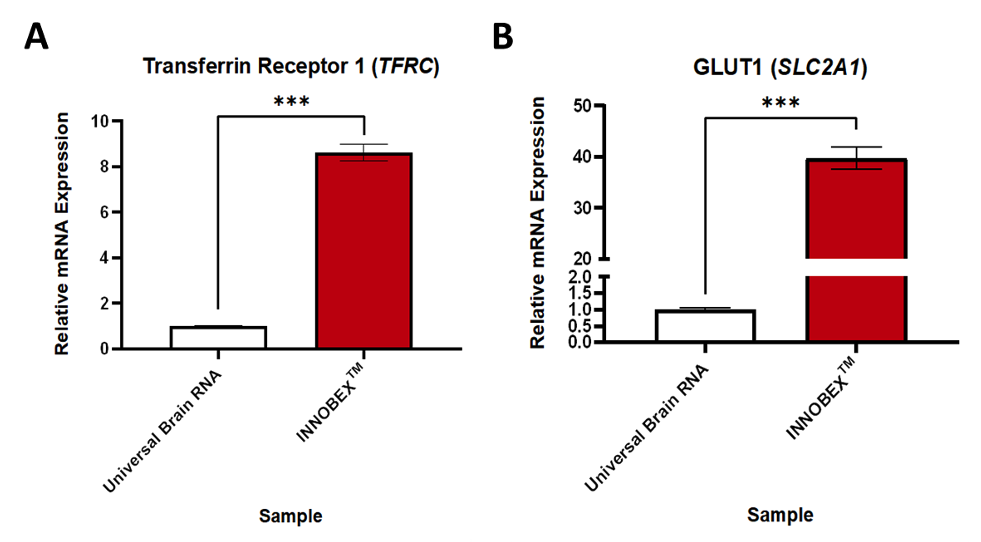
Relative expression of transporter proteins in an in vitro model of the blood-brain barrier. Gene expression data expressed relative to GAPDH and normalised to the control group. Data were analysed using unpaired t-tests. Mean ± SEM, n=3-6, ***p<0.001.
Barrier integrity and Permeability
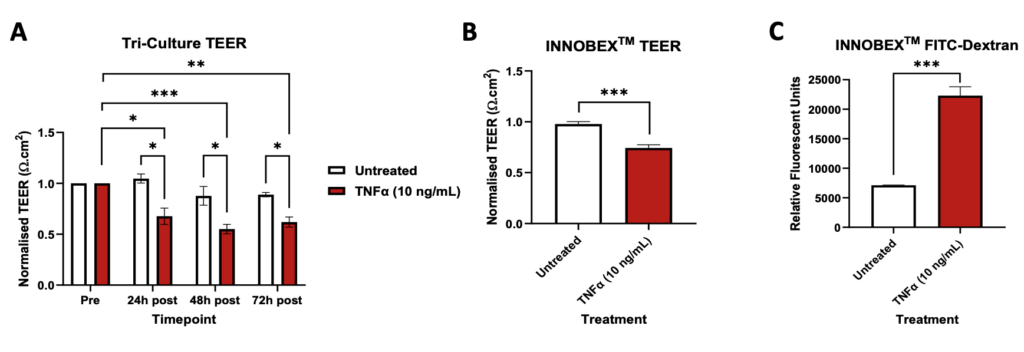
TEER and FITC-Dextran assays. TEER and FITC-Dextran data were analysed using unpaired t-tests. Mean ± SEM, Tri-Culture TEER n=2-3, INNOBEX™ TEER n=9-10, INNOBEX™ FITC-Dextran n=4, *p<0.05, **p<0.01, ***p<0.001.
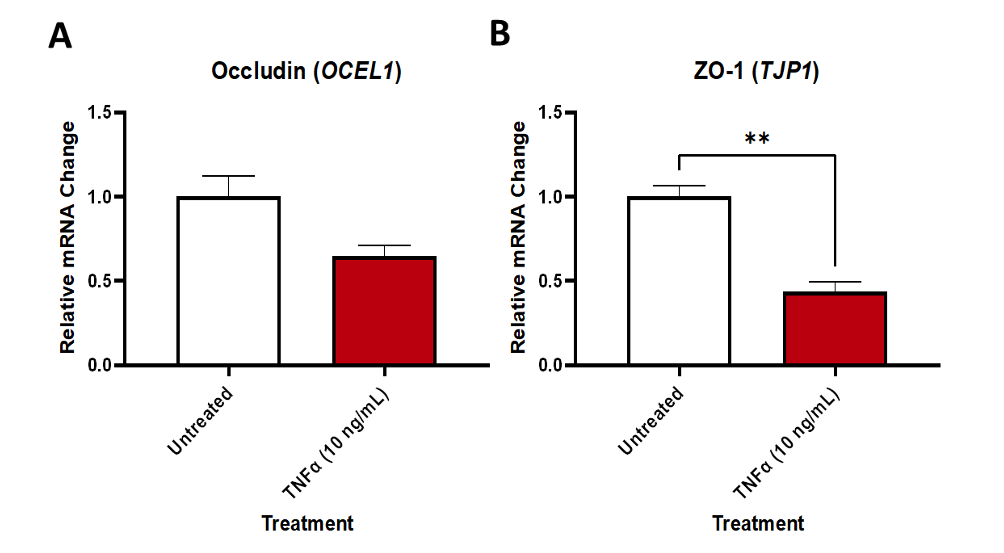
Relative expression of tight junction proteins in an in vitro model of the blood-brain barrier treated with or without TNFα. Gene expression data expressed relative to GAPDH and normalised to the control group. Data were analysed using unpaired t-tests. Mean ± SEM, n=2-3, **p<0.01.
References
- Kim J, Shin SA, Lee CS, Chung HJ. An Improved In Vitro Blood-Brain Barrier Model for the Evaluation of Drug Permeability Using Transwell with Shear Stress. Pharmaceutics. 2023 Dec 28;16(1):48. doi: 10.3390/pharmaceutics16010048.
- Johnstone M, Gearing AJ, Miller KM. A central role for astrocytes in the inflammatory response to beta-amyloid; chemokines, cytokines and reactive oxygen species are produced. J Neuroimmunol. 1999 Jan 1;93(1-2):182-93. doi: 10.1016/s0165-5728(98)00226-4.
- Spampinato SF, Merlo S, Sano Y, Kanda T, Sortino MA. Astrocytes contribute to Aβ-induced blood-brain barrier damage through activation of endothelial MMP9. J Neurochem. 2017 Aug;142(3):464-477. doi: 10.1111/jnc.14068.
- Lee HJ, Kim C, Lee SJ. Alpha-synuclein stimulation of astrocytes: Potential role for neuroinflammation and neuroprotection. Oxid Med Cell Longev. 2010 Jul-Aug;3(4):283-7. doi: 10.4161/oxim.3.4.12809.
- Meena M, Van Delen M, De Laere M, Sterkens A, Costas Romero C, Berneman Z, Cools N. Transmigration across a Steady-State Blood-Brain Barrie Induces Activation of Circulating Dendritic Cells Partly Mediated by Actin Cytoskeletal Reorganization. Membranes (Basel). 2021 Sep 13;11(9):700. doi: 10.3390/membranes11090700.
- Marchetti L, Engelhardt B. Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vasc Biol. 2020 Mar 20;2(1):H1-H18. doi: 10.1530/VB-19-0033.
- Zhu L, Yang F, Wang G, Li Q. CXC Motif Chemokine Receptor Type 4 Disrupts Blood-Brain Barrier and Promotes Brain Metastasis Through Activation of the PI3K/AKT Pathway in Lung Cancer. World Neurosurg. 2022 Oct;166:e369-e381. doi: 10.1016/j.wneu.2022.07.005.
Request a consultation with Cellomatics Biosciences today
Our experienced team of in vitro laboratory scientists will work with you to understand your molecular assay requirements and provide a bespoke project plan with a professional, flexible service and a fast turnaround time.
To request a consultation where we can discuss your exact requirements, please contact Cellomatics Biosciences.