Immuno-oncology Assays
Mixed Lymphocyte Reaction (MLR)
This reaction is relatively simple in make-up but powerful in output [1,2]. MLR relies on an allogeneic reaction; one immune cell type recognising immunological incompatibility and typically responding with a proliferative and cytokine response. Stimulator and proliferator cells isolated from two different individuals (human or rodent) are co-incubated. Proliferation in the stimulator population is inhibited (typically with mitomycin-C) then expansion of the proliferator population is measured in response to simulator exposure. The assay can be uni- or bi-directional. In the former, and more often used, cells from one of the donors will proliferate. In the latter cells from both will proliferate and will need to be identified. Proliferation can be simply measured using a non-radioactive MTT* [2] or BrdU assay. Additional endpoints such as cytokine profiling can also be undertaken adding further richness to the experimental data set.
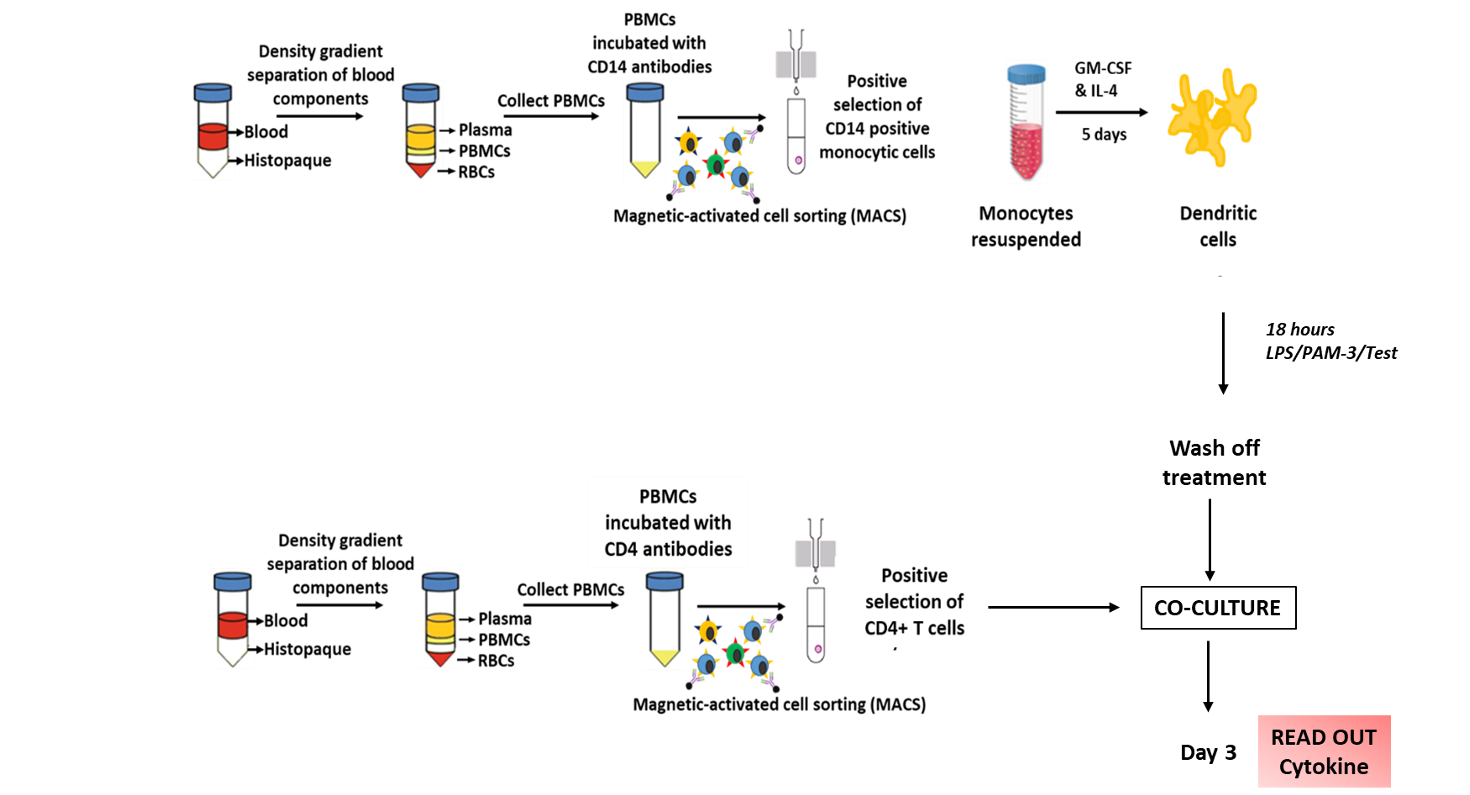
The most common use is with isolated T-cells [1] but there are assays that use PBMCs/whole blood [3]. T-cells are typically incubated with monocytes transformed into dendritic cells (DC); these are antigen presenting cells (APC). A workflow might be, isolation of monocytes, differentiation then mitomycin-C treatment followed by incubation with allogeneic T-cells. This is followed by MTT/BrdU assay for proliferation and collection of medium for multiplex cytokine assay; time courses need to be determined empirically.
MLR can be used to assess immune modulation of test compounds. Specifically, modulation of the T-cell – APC interaction. Test compounds are incubated with the T-cell proliferators prior to co-culture with the APCs. Time courses should be determined empirically and in 96-well format this assay can easily accommodate concentration response interrogation to include; potency, efficacy and also antagonist activity/affinity. MLR has been deployed to assess several immunological driven pathologies from inflammation [4] to COVID infection [5].
Cellomatics can offer human assays in volunteer isolates, this along with MTT assay and multiplex cytokine profiling are areas in which we have considerable experience. We would be happy to discuss and tailor your need so please get in touch with a member of our team.
[1]. Smialowicz RJ. (2005). Mixed Lymphocyte Reaction. In: Vohr, HW. (eds) Encyclopedic Reference of Immunotoxicology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/3-540-27806-0_1006
[2]. Knight SC, Bedford PA, Stagg AJ. (2005). Mixed leukocyte reactions, Measuring Immunity, Editors: Lotze, Thomson W, Pages: 350-360. https://doi.org/10.1016/B978-012455900-4/50292-0
[3]. Bromelow KV, Hirst W, Mendes RL, Winkley AR, Smith IE, O’Brien MER, Souberbielle BE. (2001).
Whole blood assay for assessment of the mixed lymphocyte reaction. Journal of Immunological Methods. 247(1–2):1-8. https://doi.org/10.1016/S0022-1759(00)00311-2
[4]. Seymour GJ, Boyatzis S, Powell RN. (1986). The autologous mixed lymphocyte reaction (AMLR) as a possible indicator of immunoregulation in chronic inflammatory periodontal disease. J Clin Periodontol. 13(7):639-645. https://doi.org/10.1111/j.1600-051X.1986.tb00858.x
[5]. Zhou R, To KK, Wong YC, Liu L, Zhou B, Li X, Huang H, Mo Y, Luk TY, Lau TT, Yeung P, Chan WM, Wu AK, Lung KC, Tsang OT, Leung WS, Hung IF, Yuen KY, Chen Z. (2020). Acute SARS-CoV-2 Infection Impairs Dendritic Cell and T Cell Responses. Immunity. 53(4):864-877.e5. https://doi.org/10.1016/j.immuni.2020.07.026
PBMC Proliferation
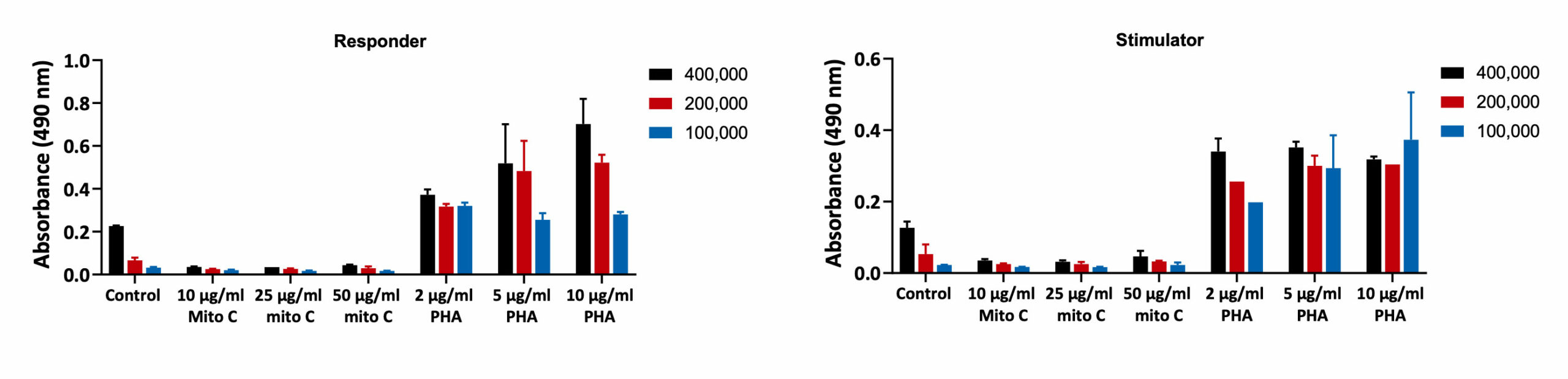
PBMCs were collected from 2 healthy donors: Donor 1 (Responder) and Donor 2 (Stimulator). PBMCs were then treated with Mitomycin for 30 minutes and seeded along with PBMCs stimulated with PHA for 5-days. The proliferation was assessed by BrdU incorporation. Mitomycin C treatment reduced the proliferation of PBMCs when compared to Untreated (Control) cells. PHA increased the proliferation of the PBMCs confirming the viability of the PBMCs after isolation (n=6±SEM).
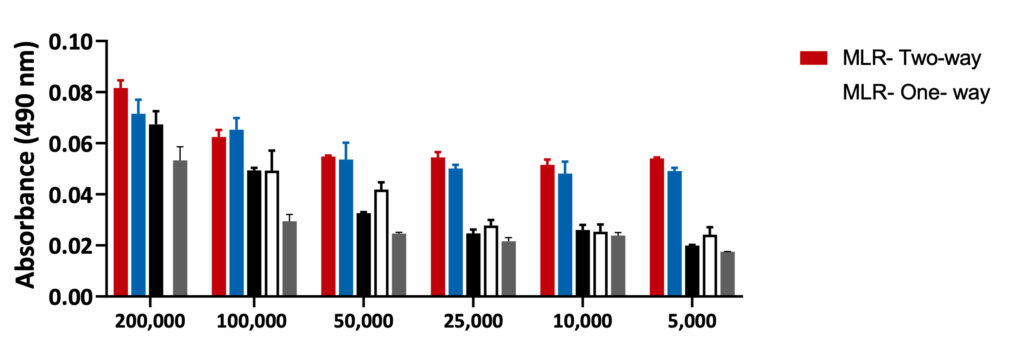
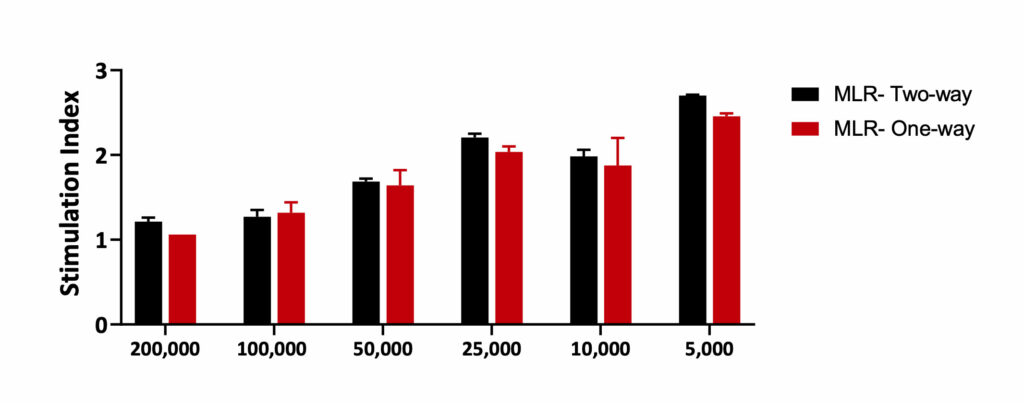
Mixed Lymphocyte Reaction- One-way and Two-way
MLR-One-way: PBMCs from stimulator were treated with Mitomycin C to inhibit proliferation. Equal numbers of the stimulator and Responder cells were then co- cultured for 5 days. Mitomycin C reduced the proliferation of Stimulator cells. Hence, any proliferation observed in the MLR-One-way is observed from the Responder cells only.
MLR- Two-way: PBMCs from both responder and stimulator were co-cultured without Mitomycin C. The proliferation was recorded from both the responder and the stimulator cells.
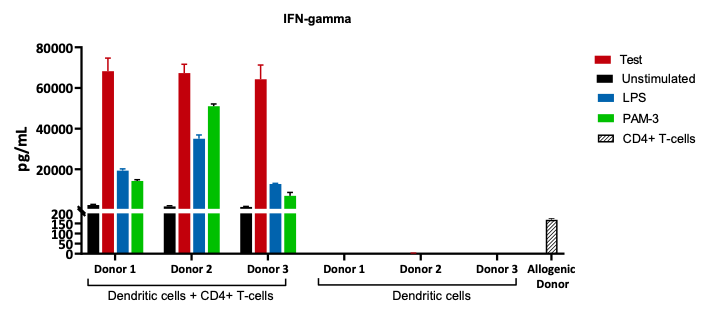
Responder cells: CD4+ T-cells from an Allogenic Donor.
Stimulator cells: Mo-DCs from 3 healthy Donors.
The supernatants after co-culture were analysed for IFN-gamma release which is an indirect measurement of T-cell activation.
Request a consultation with Cellomatics Biosciences today
Our experienced team of in vitro laboratory scientists will work with you to understand your project and provide a bespoke project plan with a professional, flexible service and a fast turnaround time.
To request a consultation where we can discuss your exact requirements, please contact Cellomatics Biosciences.