In our latest blog we discuss Non-alcoholic Steatohepatitis (NASH), how it is caused and how Cellomatics Biosciences is at the forefront of designing and validating in vitro models that replicate specific pathological features of NASH to support our clients’ drug development efforts.
Chronic liver disease is a significant cause of mortality, morbidity, and healthcare resource utilization globally (Marcellin P et al., Liver International, 2017). Modern lifestyle factors pose serious threats to liver health. High-calorie diets and decreased physical activity contribute to obesity, which increases the risk of developing type 2 diabetes. Consequently, the prevalence of non-alcoholic fatty liver disease (NAFLD) is rising (Zobair M., Younossi ZM, J Hepatology, 2018). While NAFLD, characterized by excessive fat accumulation in hepatocytes, is potentially reversible, its more severe form, non-alcoholic steatohepatitis (NASH), can progress to cirrhosis and hepatocellular carcinoma (Hallsworth K et al., JHEP Reports, 2019).
In the United States, the number of NAFLD cases is expected to rise from 83.1 million in 2015 (~25% of the population) to 100.9 million by 2030, with NASH cases increasing from 20% to 27% (Friedman SL et al., Nature Med, 2018). Patients with NASH face a higher likelihood of developing cirrhosis and end-stage liver disease, which may require liver transplantation or result in hepatocellular carcinoma. Tumours in these patients tend to be larger and less responsive to curative therapies (Piscaglia F et al., Hepatology, 2015).
There is growing interest in studying NASH due to its escalating impact on global health. However, developing effective therapeutics has proven challenging due to the disease’s complexity and the lack of noticeable symptoms in its early stages. Cellomatics Biosciences Ltd is committed to advancing research on NASH, providing validated methods to support clients’ drug development programs across various disease stages.
Non-Alcoholic Steatohepatitis (NASH)
NASH develops in approximately 20-30% of patients with NAFLD. This condition begins with steatosis, followed by progressive liver inflammation and fibrosis, ultimately leading to extensive hepatic tissue damage. Many of these patients are at high risk for cirrhosis and, in some cases, hepatocellular carcinoma.
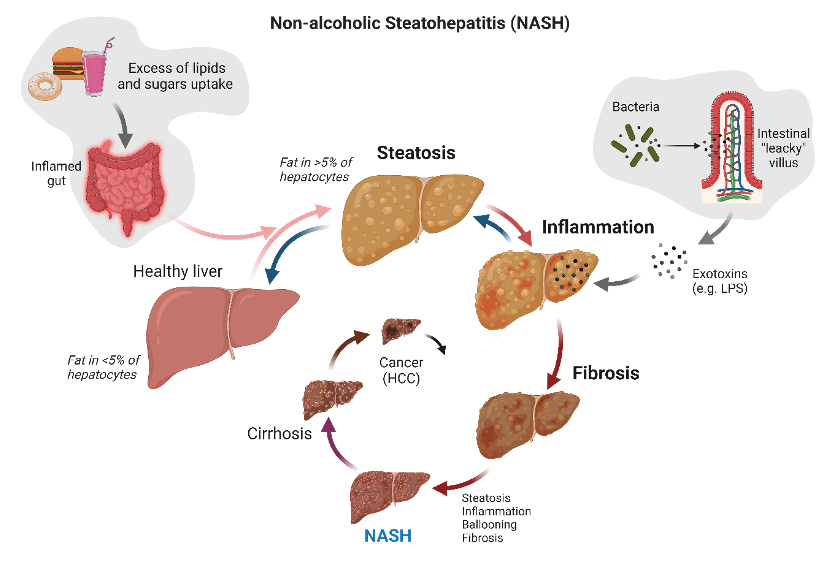
Figure 1. Summary of driving processes developing NASH and its evolution to hepatocellular carcinoma (HCC). Blue arrows indicate potential checkpoints to revert the process.
Hepatic steatosis is characterized by the accumulation of intrahepatic fats in the form of triacylglycerol (TAG), constituting at least 5% of liver weight. Under normal conditions, the liver does not store TAG; however, stressors such as obesity and high fat and carbohydrate intake can lead to imbalanced lipid metabolism and ectopic fat accumulation (Fartoux L et al., Hepatology 2005). In vitro, steatosis can be induced by exposing human hepatoma HepG2 cells to fructose and palmitate, mimicking a high-energy diet (Sasi S et al., Toxicology in Vitro, 2020). The Nile red assay can be used to assess steatosis through fluorescent imaging; in lipid-rich environments, Nile red fluoresces intensely.
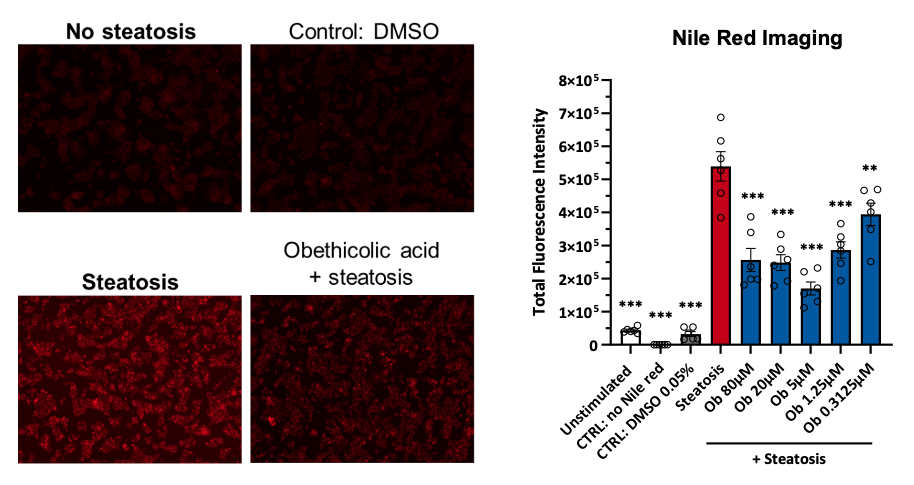
Figure 2. Induction of steatosis in HepG2 cells and Nile red staining quantification by imaging
Representative images of Nile red staining in HepG2 cells following induction of steatosis. HepG2 cells were incubated for 24 hours in relevant cell culture medium containing 100 mM of fructose and 100 µM of BSA-palmitate to induce steatosis. Different concentrations of Obethicolic acid (Ob), were used as a reference/tool compound. Data are expressed as means ± SEM; p˂0.05 considered statistically significant.
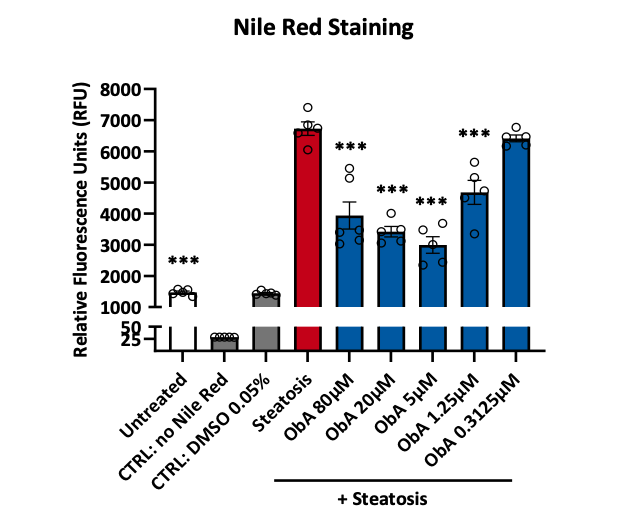
Figure 3. Induction of steatosis in HepG2 cells and quantification of Nile red staining by plate reader
HepG2 cells were plated in a 96 well plate and pre‑incubated with obethicolic acid (ObA) at the indicated concentrations before proceeding with the induction of steatosis for 24 hours. The potency of the obethicolic acid in reducing intracellular lipids was assessed measuring the fluorescence of Nile red staining on a plate reader. Data are expressed as means ± SD; p˂0.05 considered statistically significant.
Emerging evidence suggests that excessive fat and sugar intake can promote the growth of harmful gut bacteria and dysbiosis. As a result, toxins (such as LPS) may be released, triggering the immune system and leading to low-grade chronic inflammation and increased intestinal permeability (often referred to as “leaky gut”). This syndrome is common in obese individuals with glucose metabolism dysfunction and type 2 diabetes (Mishra SP et al., Gut microbiota, 2023). Elevated gut permeability facilitates the transfer of pro-inflammatory antigens (LPS), metabolites, and microbes into the bloodstream, potentially reaching the liver via the portal vein and stimulating local inflammatory responses. Hepatocytes and Kupffer cells (a type of macrophage) may react to these endotoxins, releasing pro-inflammatory cytokines that can damage surrounding liver tissue (Kessoku T et al., Frontiers in Endocrinology 2021).
In this context, inflammation plays a crucial role in the progression to NASH. To support our clients’ programs, Cellomatics Biosciences Ltd has developed a simple and reproducible co-culture model of HepG2 cells and THP-1 macrophages to evaluate potential anti-inflammatory drugs targeting early-stage NASH. Our system mimics key elements present in NASH. After stimulation with LPS, we can monitor the release of cytokines such as TNF-α, IL-1β, IL-6, and monocyte chemoattractant protein (MCP)-1, among others associated with inflammation (GM-CSF, G-CSF, CXCL4, CXCL10) (Schuster S et al., Nat Rev Gastroenterol Hepatol, 2018; Vachliotis D et al., Current Obesity Reports, 2023).
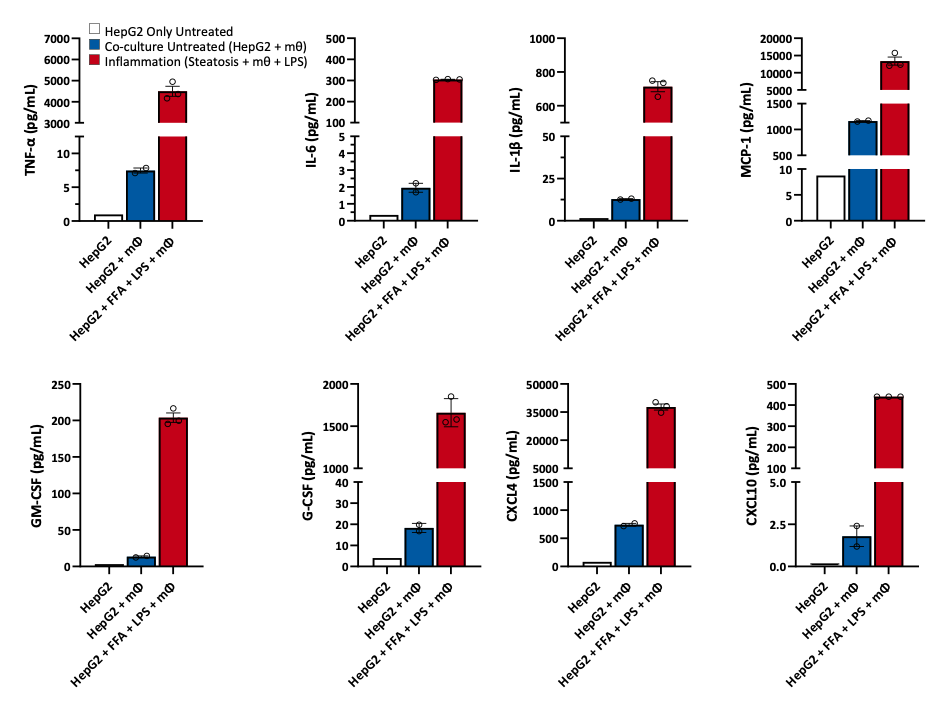
Figure 4. Quantification cytokines released in a pro‑inflammatory model of HepG2 and macrophages.
HepG2 cells were seeded in the apical transwell of 24 well plate and steatosis induced with Oleic acid and BSA‑palmitate (FFA) according to our optimised protocol (Kanmani P et al. Frontiers in Immunology 2018). Co‑culture with polarised THP‑1 cells was initiated and inflammation induced by exposing the cells to LPS (1 µg/mL) for 24 hours. Supernatants were harvested and quantification of cytokines assessed by Luminex (xMAP). Data are expressed as means ± SEM.
Macrophages are central to the pathology of NASH. Attracted by the presence of bacterial components, these cells infiltrate liver tissue and produce toxic effector molecules (reactive oxygen species, or ROS), which contribute to tissue damage and fibrosis. In our co-culture model of steatosis (HepG2 + FFA) with LPS-pulsed macrophages, we observed a significant increase in FGF2 levels. FGF2 is upregulated in chronic liver disease and mediates fibrogenesis by activating hepatic stellate cells, linking extracellular matrix modulation and carcinogenesis to NASH (Ocker M., W J Gastroenterology, 2020).
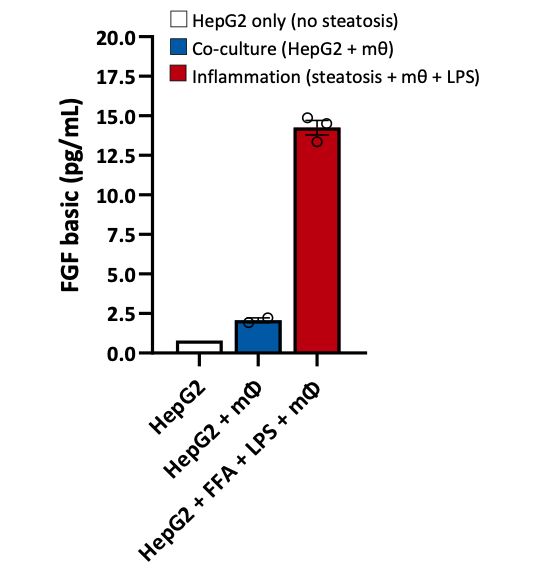
Figure 5. Quantification of FGF in the co‑culture model of HepG2 and macrophages.
HepG2 cells were seeded in the apical transwell of 24 well plate and steatosis induced with Oleic acid and BSA‑palmitate (FFA) according to our optimised protocol (Kanmani P et al. Frontiers in Immunology 2018). Co‑culture with polarised THP‑1 cells was initiated and inflammation induced by exposing the cells to LPS (1 µg/mL) for 24 hours. Supernatants were harvested and quantification of cytokines assessed by Luminex (xMAP). Data are expressed as means ± SEM.
Liver fibrosis is an altered process characterized by excessive collagen and matrix deposition, leading to the replacement of liver parenchyma, increased stiffness, and vascular resistance. This progression results in liver failure and complications like cirrhosis and cancer. Hepatic stellate cells (HSC) are primarily responsible for organizing the structural changes associated with a fibrotic or cirrhotic NASH liver, particularly the deposition of type I collagen in the extracellular matrix. During chronic liver injury, transforming growth factor (TGF-β) is recruited from ECM deposits. Stimulation of HepG2 cells with TGF-β increases the expression of fibrosis biomarkers (e.g., collagen type I alpha 1, COL1A1, and alpha-smooth muscle actin, α-SMA).
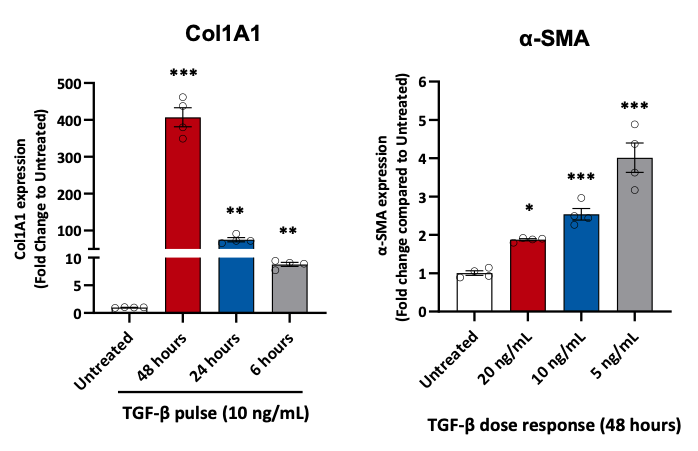
Figure 6. Fibrotic markers in HepG2 cells stimulated with TGF-β.
Gene expression analysis of collagen type I alpha 1 (COL1A1) and α-smooth-muscle actin (α-SMA) in HepG2 cells stimulated with TGF-β. Data are expressed as means ± SEM; p˂0.05 considered statistically significant.
Hepatic fibrosis is triggered by multiple dysfunctions converging on liver tissue. Mitochondrial dysfunctions, which induce oxidative stress, play a key role in this process. At Cellomatics Biosciences Ltd, we have studied a common antioxidant and validated a fast and reliable assay to monitor intracellular ROS levels in HepG2 cells.

Figure 7. Levels of intracellular reactive oxygen species in HepG2 cells.
Quantification of ROS in HepG2 cells following oxidative stress induced by validated ROS inducers (antimycin A and oxygen peroxide – H2O2). Data are expressed as means ± SEM. One-way ANOVA, p˂0.05 considered statistically significant (Reference = antimycin A standard, NAC = N‑acetylcysteine).
N-acetylcysteine (NAC) is an antioxidant that may help reduce inflammation. It is metabolized into cysteine, which is then transformed into glutathione, a vital antioxidant for immune function and detoxification. Pretreatment of HepG2 cells with NAC decreased intracellular ROS levels induced by agents like antimycin A or hydrogen peroxide. While NAC is associated with various health benefits, its efficacy needs further scientific validation. Here, we confirm, using a simple and validated fluorescent assay, that NAC is a promising candidate for reducing ROS and inflammation in livers affected by NASH (Baumgardner JN et al., ASN 2008).
The liver plays a central role in numerous biological functions, impacting nearly every system in the body. The consumption of high-calorie foods and reduced physical activity have contributed to rising obesity and type 2 diabetes rates, increasing the risk of chronic diseases like NASH. Despite its prevalence and clinical significance, no licensed therapies exist for NASH, due to the lack of reliable models for testing drug candidates. However, significant progress has been made in recent years, and Cellomatics Biosciences Ltd is at the forefront of designing and validating in vitro models that replicate specific pathological features of NASH to support our clients’ drug development efforts.
If you would like to learn more about our models and how we can support your drug discovery journey, please do not hesitate to get in touch.
References
- Zobair M. Younossi. Non-alcoholic fatty liver disease – A global public health perspective. Journal of Hepatology, 2018
- Hallsworth K et al. Lifestyle modification in NAFLD/NASH: Facts and figures. JHEP Reports, 2019.
- Kessoku T et al. Endotoxins and non‑alcoholic fatty liver disease. Frontiers in Endocrinology, 2021
- Piscaglia F et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease A. Hepatology, 2015
- Ströbel S et al. A 3D primary human cell‑based in vitro model of non‑alcoholic steatohepatitis for efficacy testing of clinical drug candidates. Scientific Reports, 2021.