Inflammation Assays
Other Models
Our team has developed other models including
- Vascular permeability assay
- Human dermal papilla cell based inflammatory model – Alopecia
- Glucose Metabolism and oxidative stress
Vascular Permeability
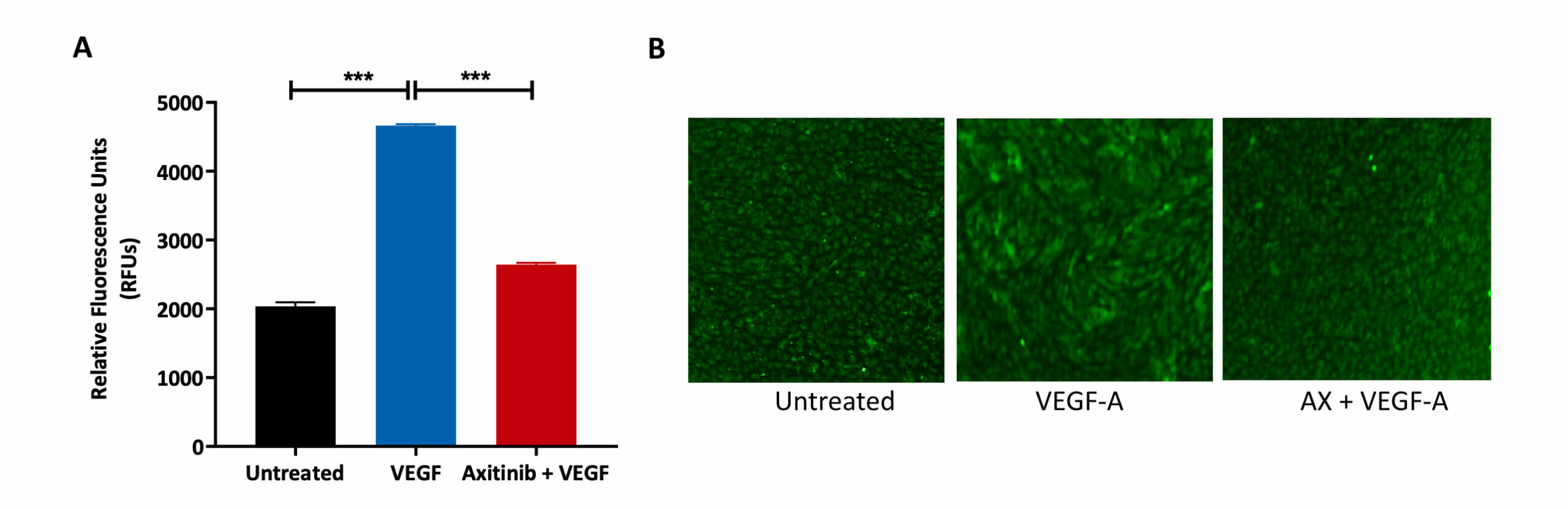
HUVECs were allowed to form a monolayer, prior to treatment with the VEGFR inhibitor Axitinib (AX) and VEGF-A. Untreated control was also included as part of the analysis.
(A) FITC-Dextran mediated measure of permeability. VEGF-A treatment resulted in an increase in monolayer permeability as indicated by the increase in fluorescence output (RFU) relative to the untreated condition. Pre-treatment with AX reduced the fluorescence output despite the addition of VEGF-A, showing that AX reverses the permeabilisation effect of VEGF-A.
(B) Calcein-AM staining of HUVEC cells after drug treatment. A tight HUVEC monolayer was formed in the untreated condition but upon treatment with VEGF-A, the cellular arrangement has changed indicating movement and motility. Pre-treatment with AX maintained the integrity of the monolayer cell arrangement as that found in the untreated condition.
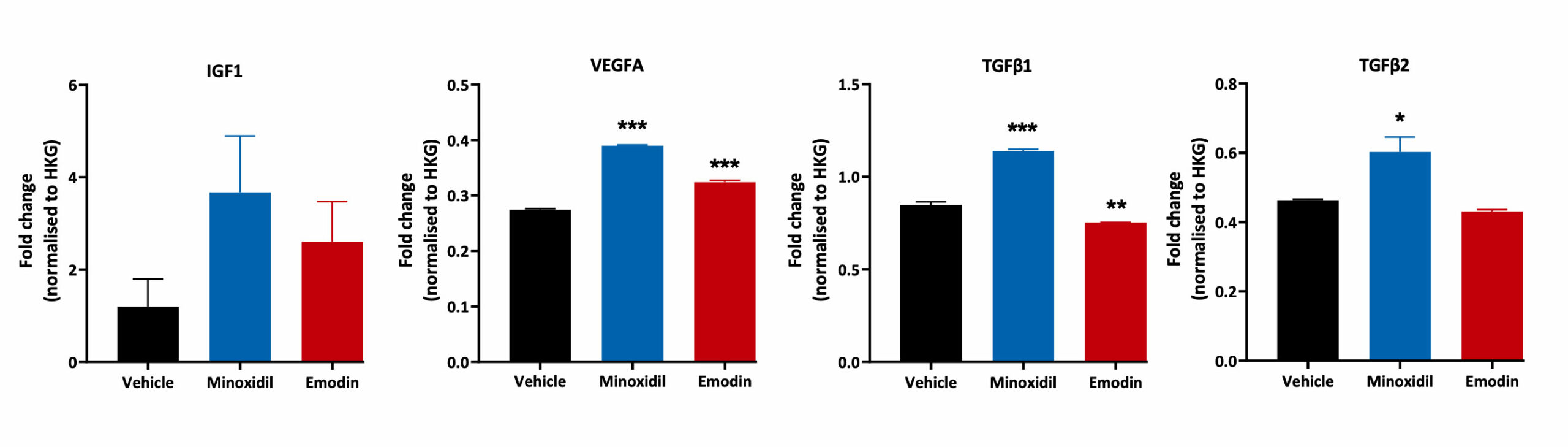
Human Dermal Papilla Cells and Inflammation
Human Follicle Dermal Papilla Cells were treated with Minoxidil and Emodin for 48 hours. Cell lysates were then analysed for TGFβ1, TGFβ2, IGF1 and VEGFA gene expression using Quantigene™ Multiplex Assay. Changes in expression levels for each treatment condition were normalised to the house keeping gene and compared to the vehicle control (***p<0.001, **p<0.01, *<p0.05; n=3±SEM).
Glucose Metabolism and oxidative stress
C2C12 are an immortalised mouse myoblast cell line. They a well-known model for metabolic disorders and are used to look at mitochondrial metabolism as well as glucose metabolism. They are a well documented at looking into the effects of ROS related oxidative stress.
Reactive oxygen species (ROS) are essential in a number of biological processes including metabolism, differentiation and proliferation. Oxidative stress is caused by the elevation of intracellular levels of ROS, which can cause damage to DNA, proteins, and lipids. Oxidative damage from ROS has been noted in several disease areas including cancer, aging, and metabolic conditions. It is important therefore to understand the role of ROS in a multitude of disease areas. ROS can be quantified in vitro, in live cells, by using the fluorogenic dye 2’,7’ –dichlorofluorescin diacetate (DCFDA) which diffuses in the cells and is converted to 2’, 7’ –dichlorofluorescein and measured by fluorescence spectroscopy .
Glucose Oxidase which is known to increase ROS through catalysing glucose to hydrogen peroxide was used as a control along with known ROS inducers tert-Butyl hydroperoxide (TBHP) and Palmitic acid (PA). PP2, a known inhibitor of Palmitic acid, was used to decrease the effect of PA on the release of ROS.

ROS Assay: GOx dose response
Prior to treatment C2C12 were differentiated to form mature myotubles and then incubated with DCFDA. C2C12 cells were treated with GOx at 7 concentrations and incubated 37°C in a humidified incubator. The fluorescence was measured by plate reader at 1 and 4 hours post treatment

Request a consultation with Cellomatics Biosciences today
Our experienced team of in vitro laboratory scientists will work with you to understand your project and provide a bespoke project plan with a professional, flexible service and a fast turnaround time.
To request a consultation where we can discuss your exact requirements, please contact Cellomatics Biosciences.