PBMC Based Assays
Peripheral blood mononuclear cells (PBMC) provide selective responses to the immune system and are the major immune cells in the human body. PBMCs include lymphocytes (T cells, B cells, and NK cells), monocytes, and dendritic cells. PBMC based assays explore the role of these cells in immune modulation, cell proliferation/cytotoxicity and their role in targeting specific cancer cells.
The expert team at Cellomatics can support with PBMC assays within a variety of projects.
PBMCs – anti-CD3 stimulation
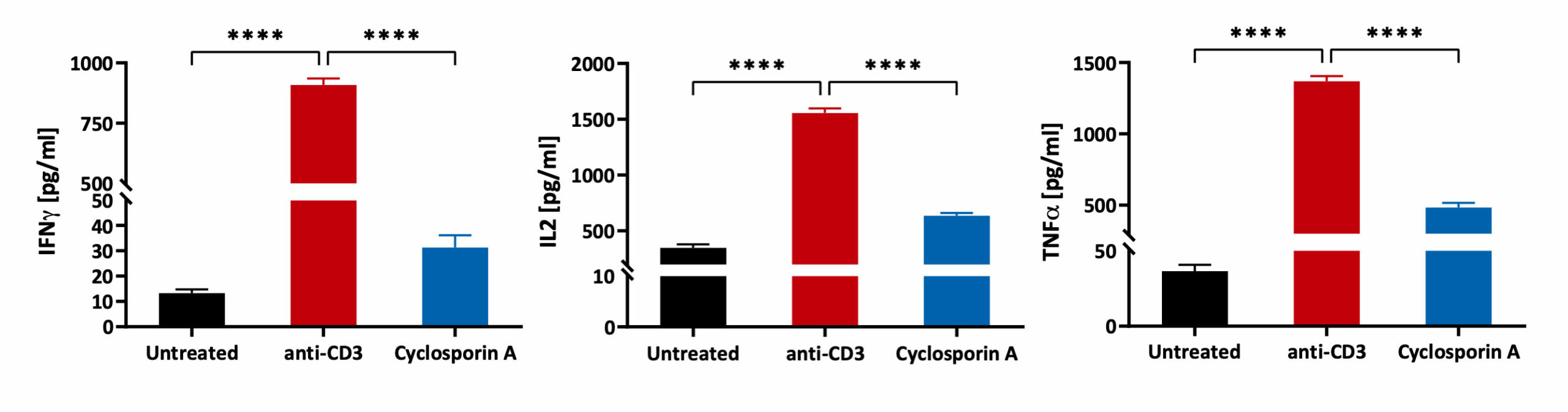
Freshly isolated Peripheral Blood Mononuclear cells (PBMCs) were seeded onto anti-CD3 coated U-bottom plates and further stimulated with soluble anti-CD28 for 48 hours. PBMCs were also treated with Cyclosporin A prior to stimulation. Isotype of anti-CD3 was used as a background control. Supernatants were analysed for inflammatory markers using a Luminex Multiplex Assay (n=3±SEM; *p<0.05; ****p<0.0001).
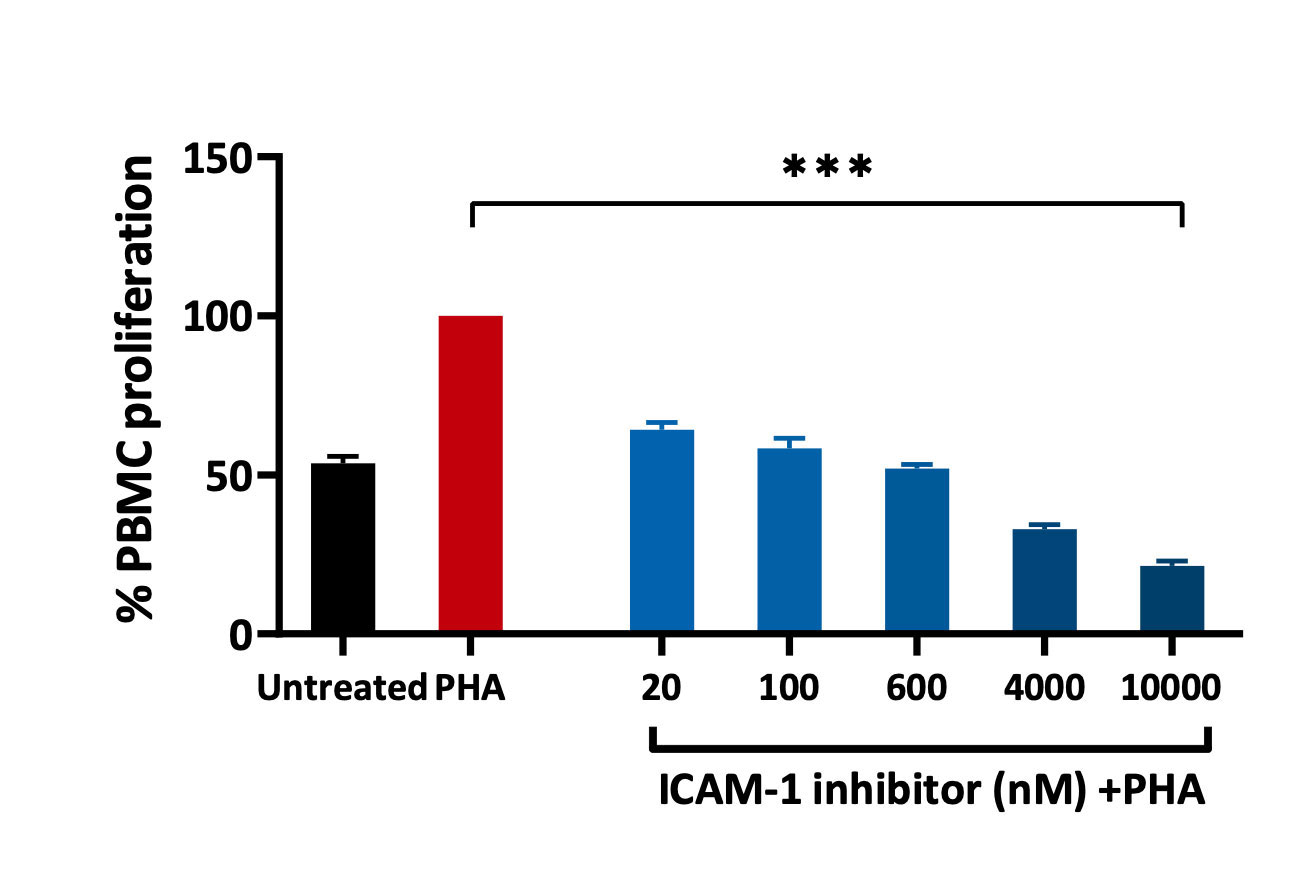
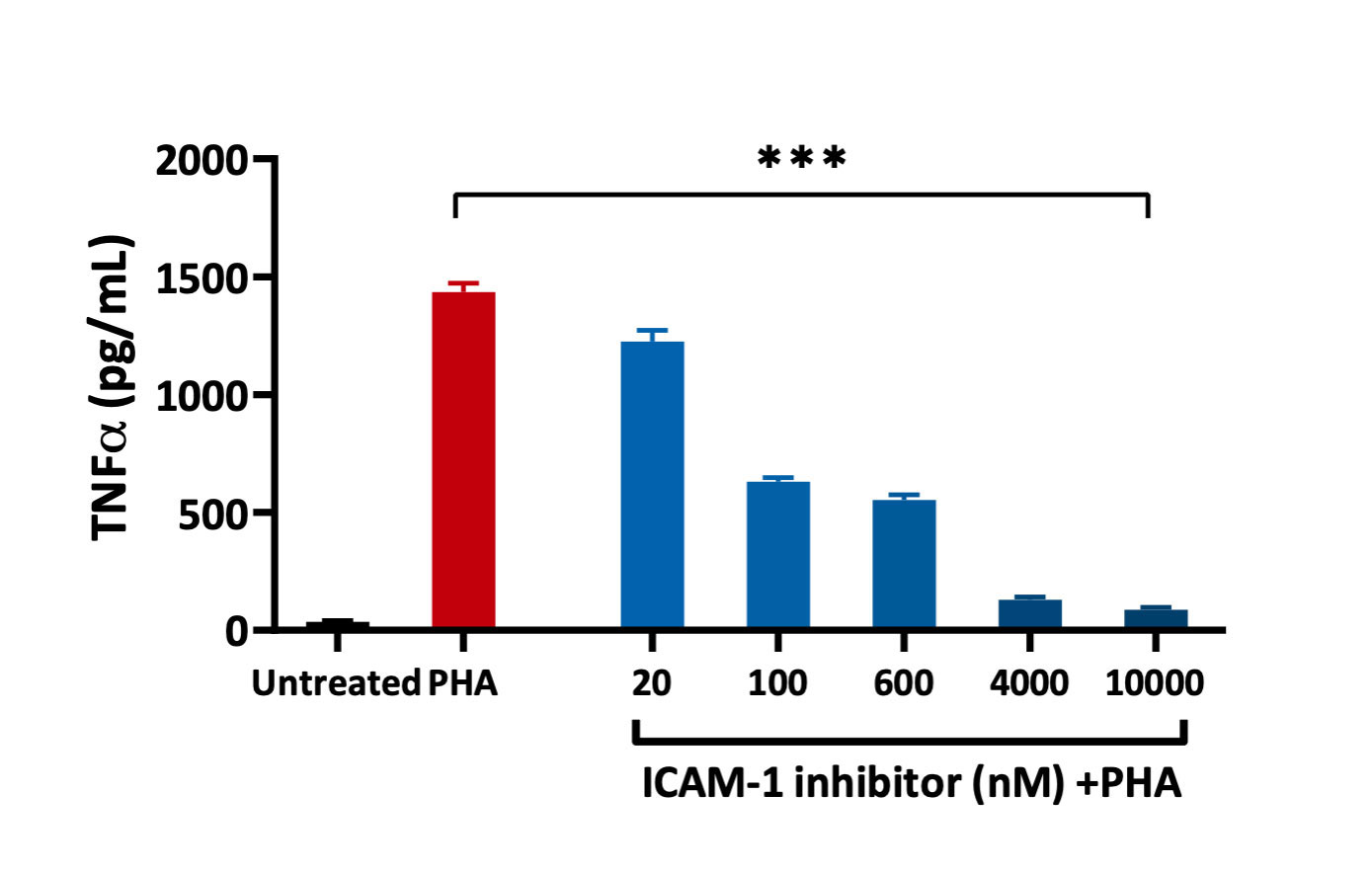
PBMCs – PHA stimulation
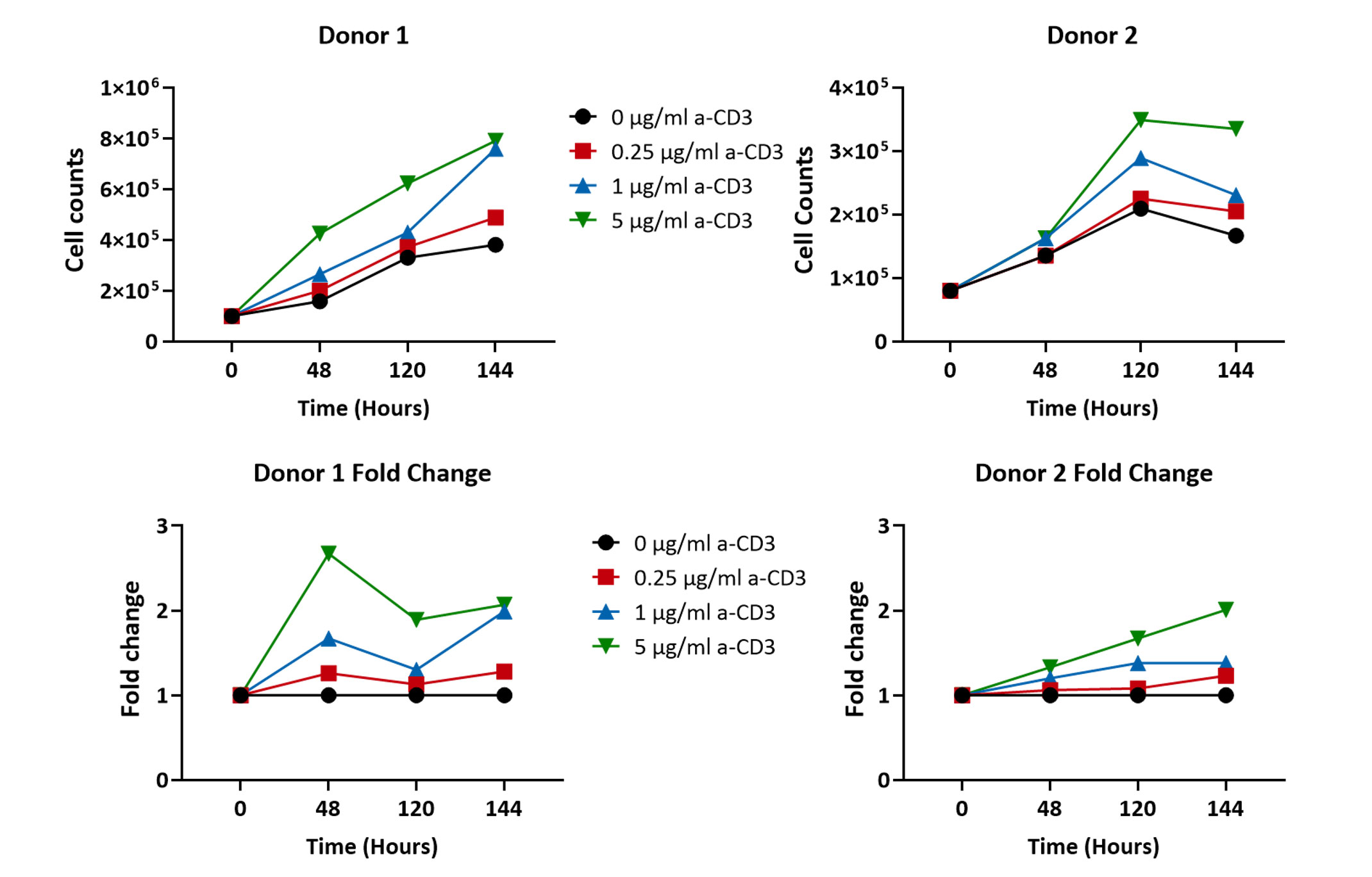
PBMCs proliferate in response to anti-CD3 stimulation
PBMCs from 2 different healthy volunteers were stimulated with increasing concentrations of anti-CD3 antibody (0 µg/ml, 0.25 µg/ml, 1 µg/ml and 5 µg/ml) over the period of 144 hours (6 days) and the cell proliferation was recorded. A concentration dependent increase in PBMC cell counts was observed after 48 hours of stimulation (n=5±SEM).
PBMC – CD3/CD28 stimulation
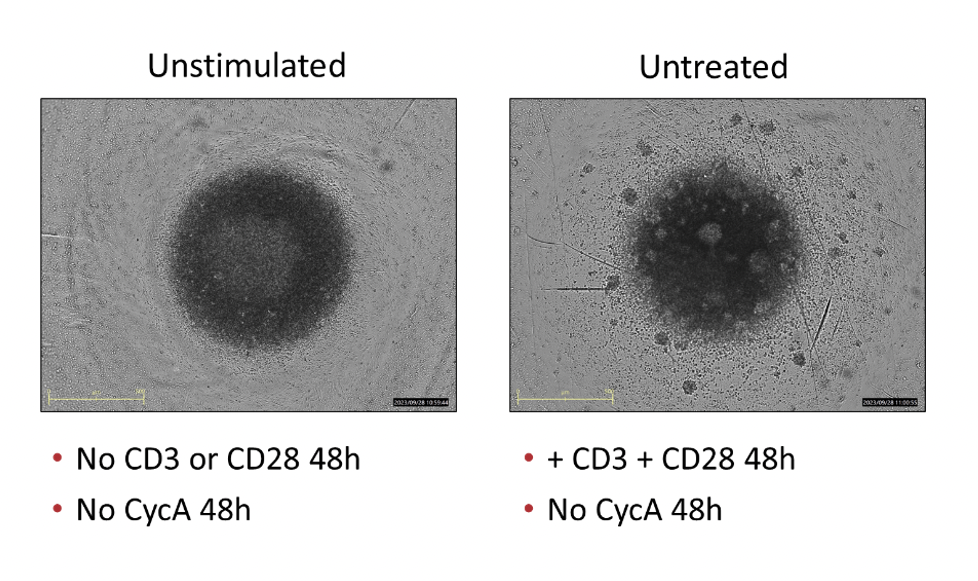
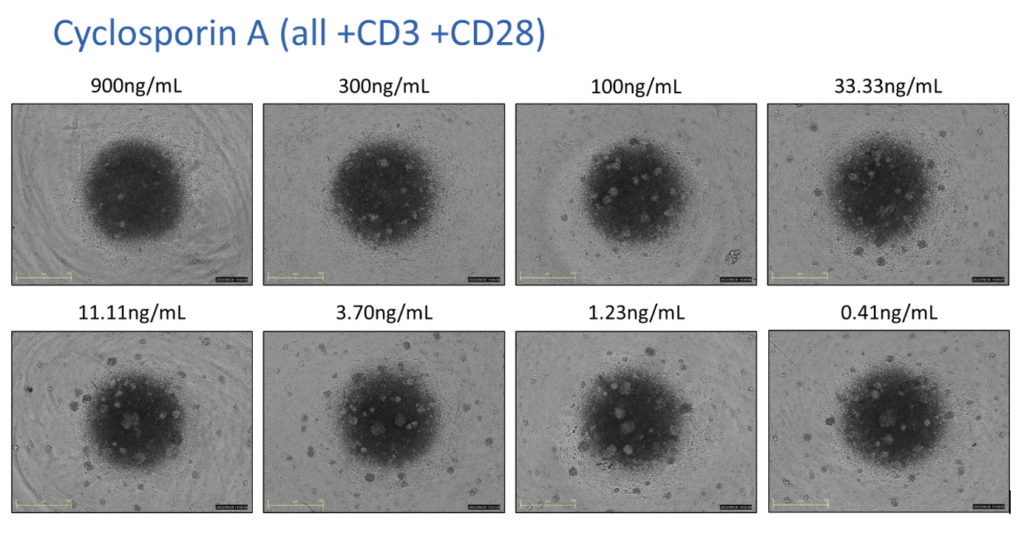

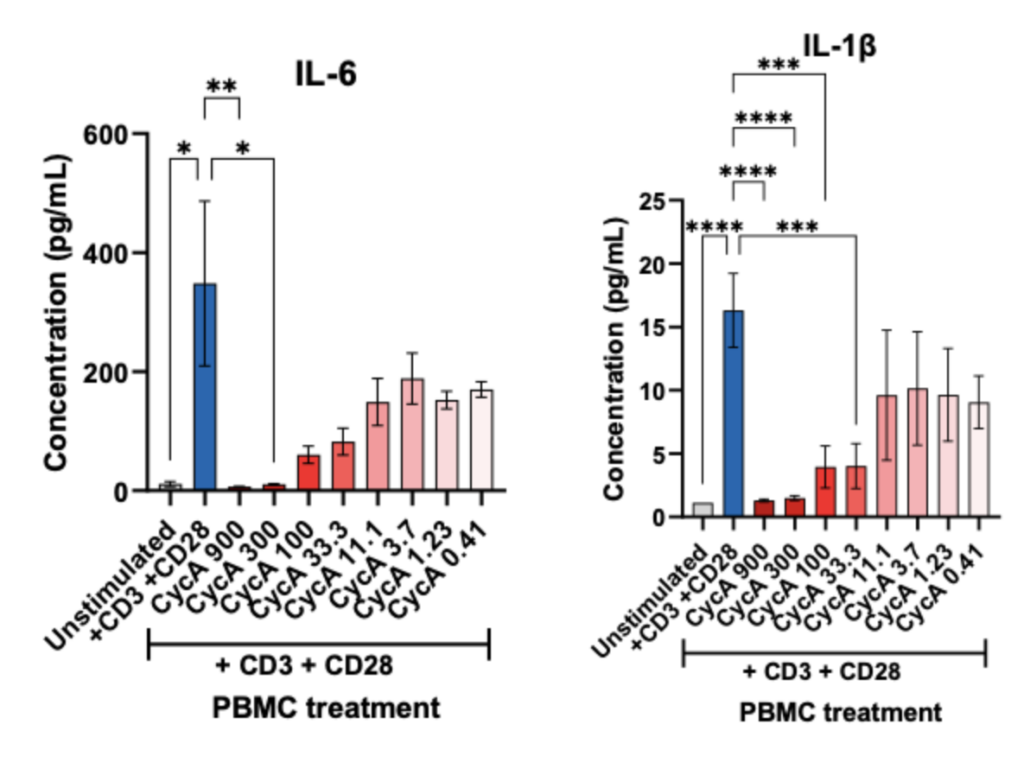
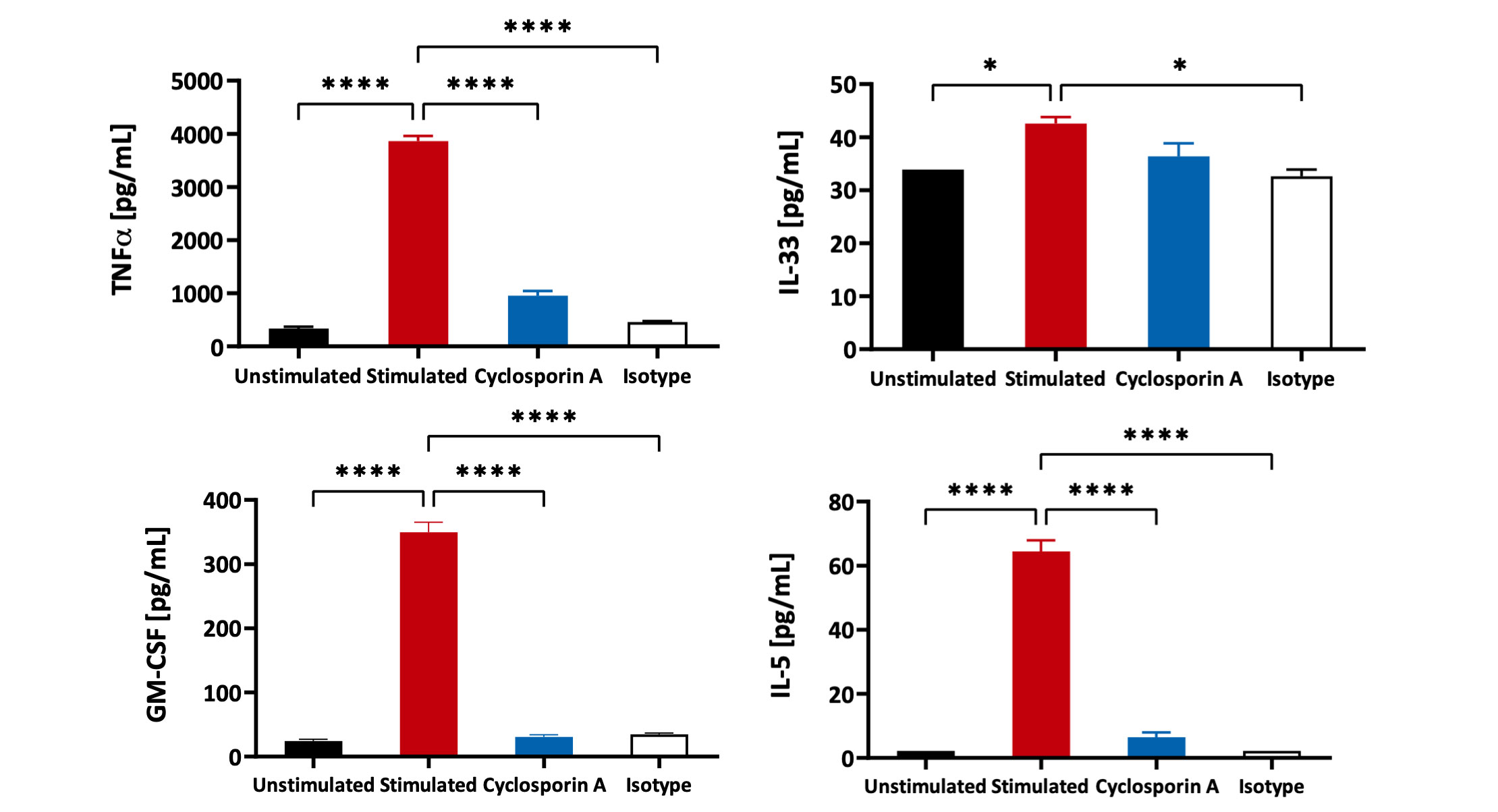
PBMCs – anti-CD3/anti-CD28 stimulation Freshly isolated Peripheral Blood Mononuclear cells (PBMCs) were seeded onto anti-CD3 coated U-bottom plates and further stimulated with soluble anti-CD28 for 48 hours. PBMCs were also treated with Cyclosporin A in a 8 concentration-response-curve prior to stimulation. Supernatants were analysed for inflammatory markers using Luminex Multiplex Assay (n=3±SEM; *p<0.05; ****p<0.0001).
PBMCs – anti-CD3/anti-CD28 stimulation
PBMCs – anti-CD3/anti-CD28 stimulation Freshly isolated Peripheral Blood Mononuclear cells (PBMCs) were seeded onto anti-CD3 coated U-bottom plates and further stimulated with soluble anti-CD28 for 48 hours. PBMCs were also treated with Cyclosporin A prior to stimulation. Isotype of anti-CD3 was used as a background control. Supernatants were analysed for inflammatory markers using Luminex Multiplex Assay (n=3±SEM; *p<0.05; ****p<0.0001
PBMCs – anti-CD3/anti-CD28 stimulation for 72 hours
Freshly isolated Peripheral Blood Mononuclear cells (PBMCs) were seeded onto anti-CD3 coated U-bottom plates and further stimulated with soluble anti-CD28 for 24, 48 and 72 hours. PBMCs were also treated with Cyclosporin prior to stimulation. Isotype of anti-CD3 was used as a background control. Supernatants were analysed for inflammatory markers using Luminex Multiplex Assay (n=5±SEM; **p<0.01; ***p<0.001; ****p<0.0001).
PBMCs - LPS stimulation


Freshly isolated Peripheral Blood Mononuclear cells (PBMCs) were seeded onto anti-CD3 coated U-bottom plates and further stimulated with LPS for 48 hours in the presence or absence of Dexamethasone. Supernatants were analysed for inflammatory markers using a Luminex Multiplex Assay (n=3±SEM; *p<0.05; ****p<0.0001).
PBMCs - LPS stimulation

Freshly isolated Peripheral Blood Mononuclear cells (PBMCs) from 3 donors were stimulated with LPS for 24 hours in the presence or absence of Dexamethasone. Supernatants were analysed for inflammatory markers using Luminex Multiplex Assay. There was a significant increase in TNF-α expression with 100 ng/mL LPS (***p<0.001). Significant differences were identified using a two-way ANOVA followed by a Šídák’s multiple comparisons test.

Freshly isolated Peripheral Blood Mononuclear cells (PBMCs) from 3 donors were stimulated with LPS for 24 hours in the presence or absence of Dexamethasone. Supernatants were analysed for inflammatory markers using Luminex Multiplex Assay. There was a significant increase in IL6 expression with 100 ng/mL LPS (***p<0.001). Significant differences were identified using a two-way ANOVA followed by a Šídák’s multiple comparisons test.
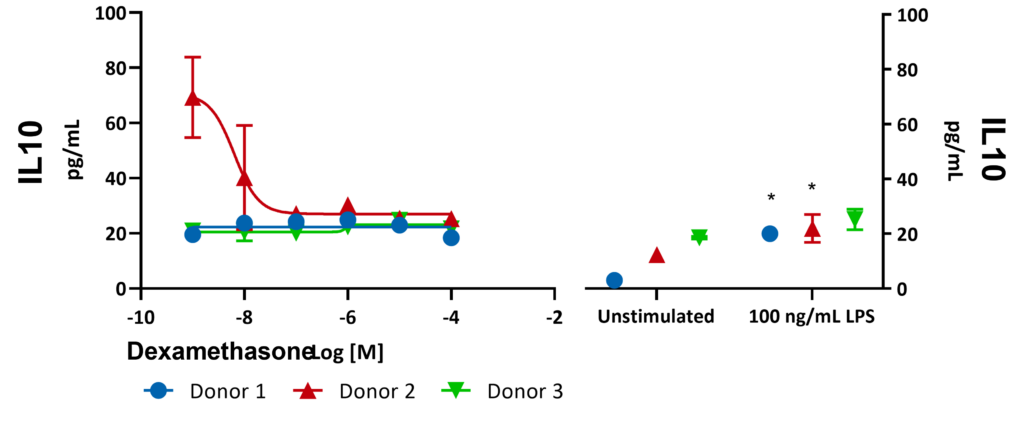
Freshly isolated Peripheral Blood Mononuclear cells (PBMCs) from 3 donors were stimulated with LPS for 24 hours in the presence or absence of Dexamethasone. Supernatants were analysed for inflammatory markers using Luminex Multiplex Assay. There was a significant increase in IL10 expression with 100 ng/mL LPS (***p<0.001). Significant differences were identified using a two-way ANOVA followed by a Šídák’s multiple comparisons test.
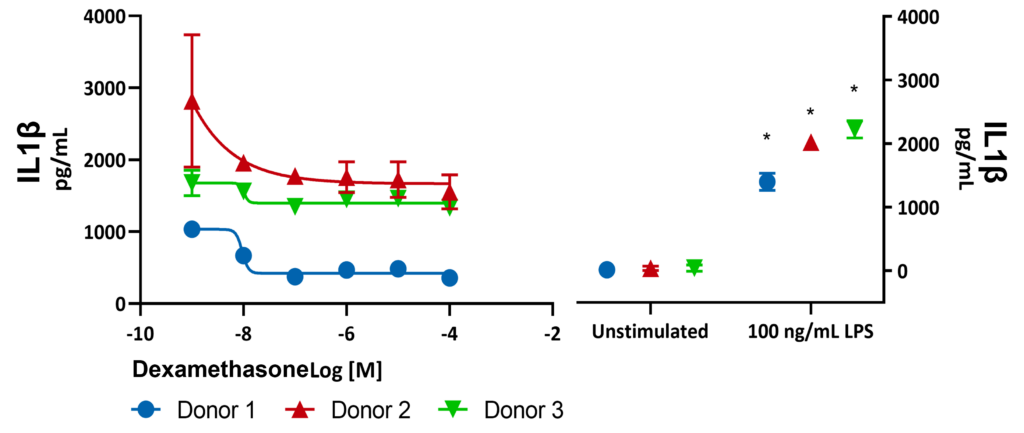
Freshly isolated Peripheral Blood Mononuclear cells (PBMCs) from 3 donors were stimulated with LPS for 24 hours in the presence or absence of Dexamethasone. Supernatants were analysed for inflammatory markers using Luminex Multiplex Assay. There was a significant increase in IL1β expression with 100 ng/mL LPS (***p<0.001). Significant differences were identified using a two-way ANOVA followed by a Šídák’s multiple comparisons test.
PBMC – NOD2 signalling
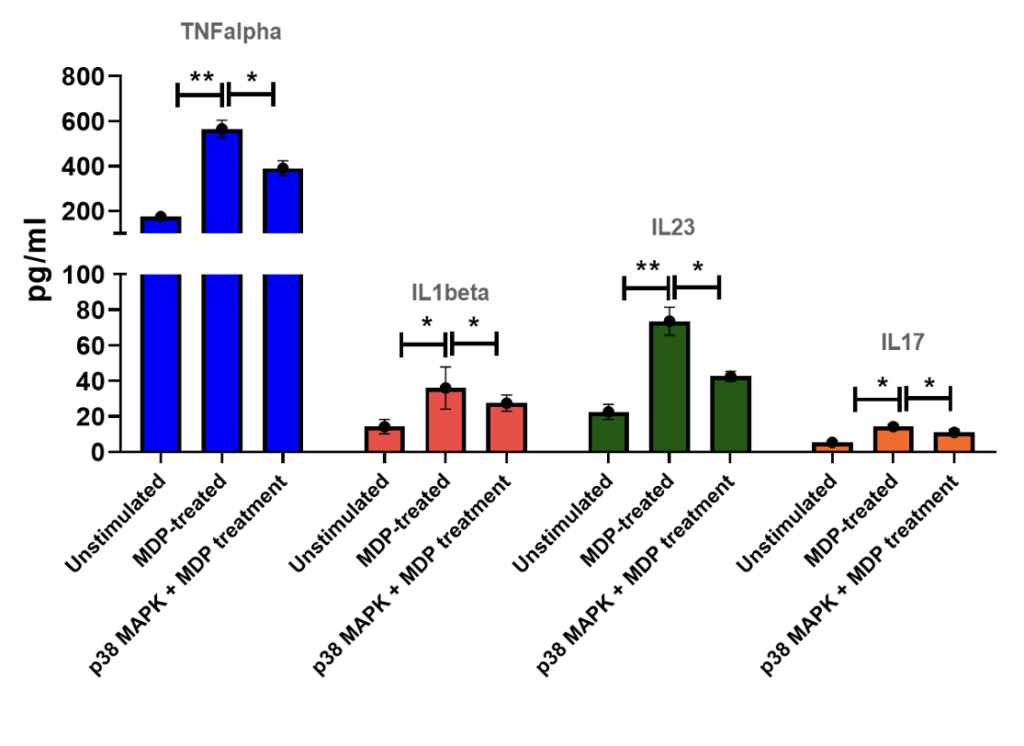
PBMCs + MDP (muramyl dipeptide) stimulation Levels of TNF alpha, IL1beta, IL-23 and IL-17 in PBMC supernatants of healthy volunteers following stimulation with MDP for 24 hrs in the absence or presence of p38MAPK inhibitor. Results are expressed in pg/mL.
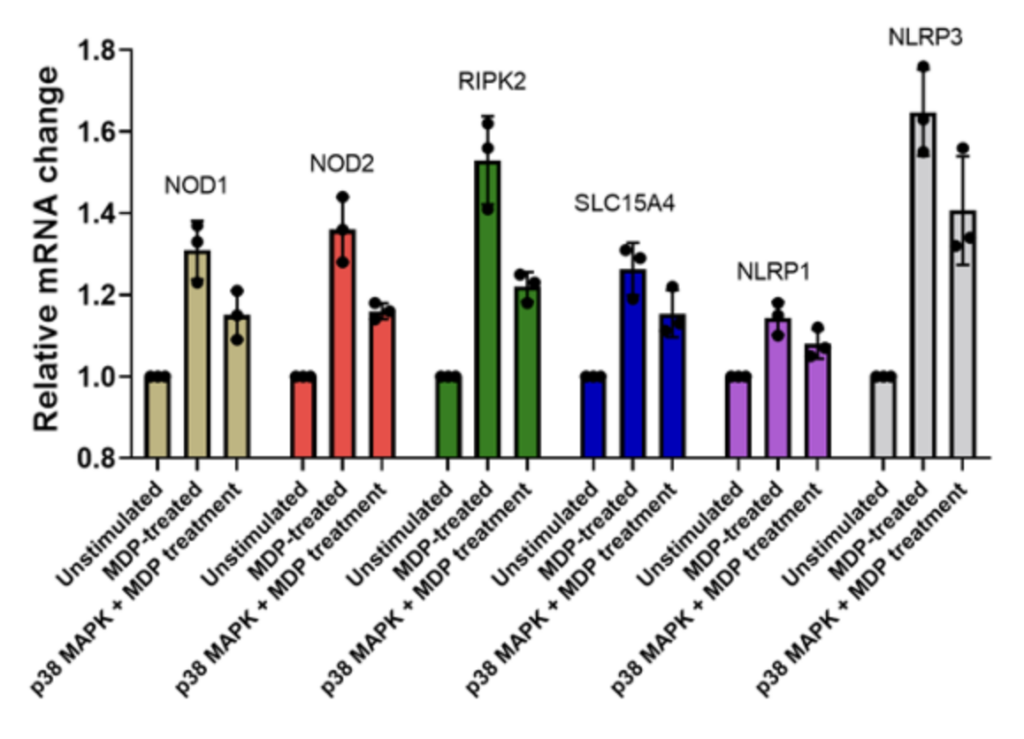
PBMCs + MDP (muramyl dipeptide) stimulation Relative mRNA levels of NOD1, NOD2, RIPK2, SLC15A4, NLRP1 and NLRP3 following stimulation of PBMCs (from a healthy volunteer) with MDP in the absence or presence of p38MAPK inhibitor.
PBMC – Inflammasome activation

PBMCs from healthy domors were stimulated with LPS alone, LPS+ATP in the presence or absence of NLRP3 inhibitor. Supernatants were analysed for NLRP3 and IL-1β using ELISA. Statistical analysis was performed using a 2WAY ANOVA, followed by Šídák’s multiple comparisons test for each donor independently. All treatment groups were compared to the untreated group (n=3 ± SEM; ***p<0.001, **p<0.01). Notably, the combination of LPS + ATP for 1 hr with 10µM NLRP3 inhibitor significantly reduced the expression of both NLRP3 and IL-1β in PBMCs from healthy donors.


PBMCs from healthy donors were stimulated with LPS alone, LPS+ATP in the presence or absence of NLRP3 inhibitor. Supernatants were analyzed using ELISA for NLRP3, IL-1β and Caspase 1. Data was subjected to statistical analysis using an ordinary one-way ANOVA, followed by a Dunnett’s multiple comparisons test for each donor independently. All treatment groups were compared to the LPS+ATP group (n=3 ± SEM; ***p<0.001, **p<0.01). The combination of 10µM-1µM NLRP3 inhibitor with LPS and ATP significantly reduced the expression of both NLRP3 and IL-1β in healthy PBMCs.
PBMC – ADCC (tumour cell killing)
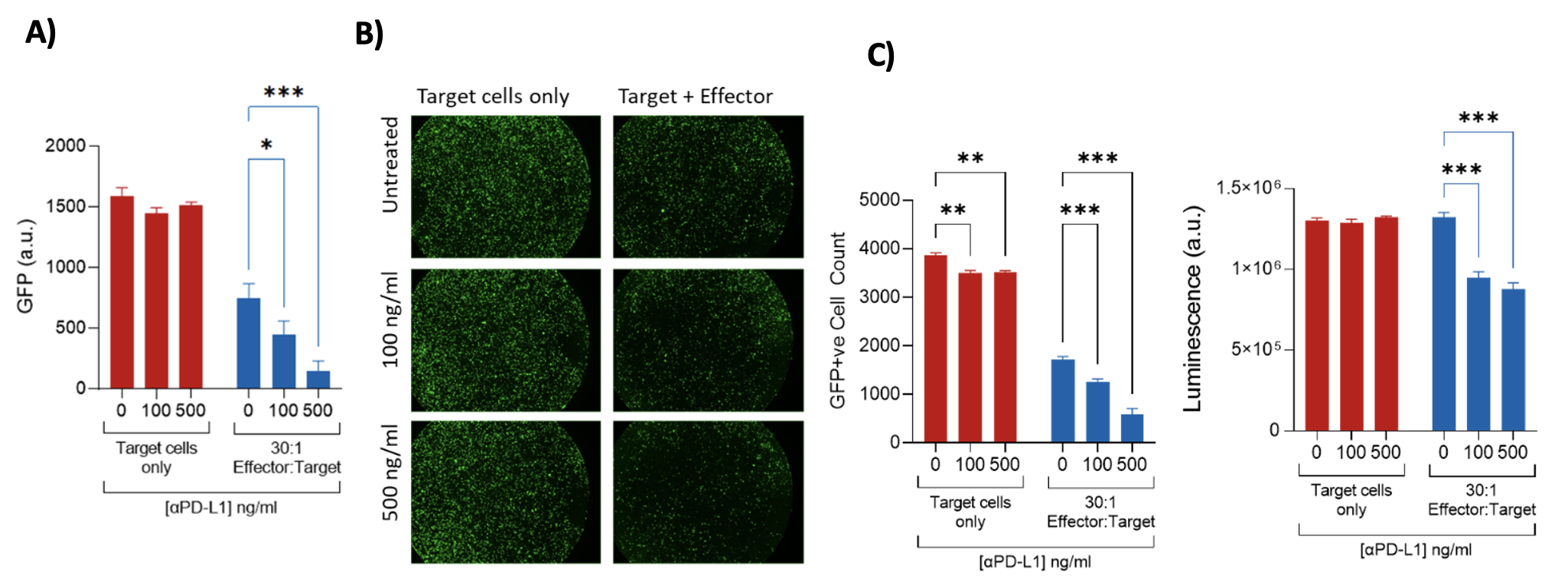
Assessment of ADCC via GFP release – MB231 (target cells)/PBMCs(effector cells) MB231 target cells were lipofected with a GFP expression plasmid and incubated with an antibody targeting human PD-L1-hlgG1fut. Target cells were subsequently incubated for 24 h with PBMCs (Effector cells) at a ratio of 30:1. ADCC was assessed by means of loss of intracellular GFP (GFP levels were measured on a plate reader, and the number of GFP+ve cells quantified by image analysis) and loss of viability (CellTiter Glo assay). A) A loss of intracellular GFP (demonstrating ADCC of GFP+ve Target cells) is induced by the Effector cells and is significantly increased by the antibody; B) Visualisation and subsequent image analysis demonstrate a loss of GFP+ve Target cells in the presence of the Effector cells, which is significantly enhanced by incubation with the antibody: C) A loss of viability occurs following incubation of Target cells with the antibody and Effector cells.
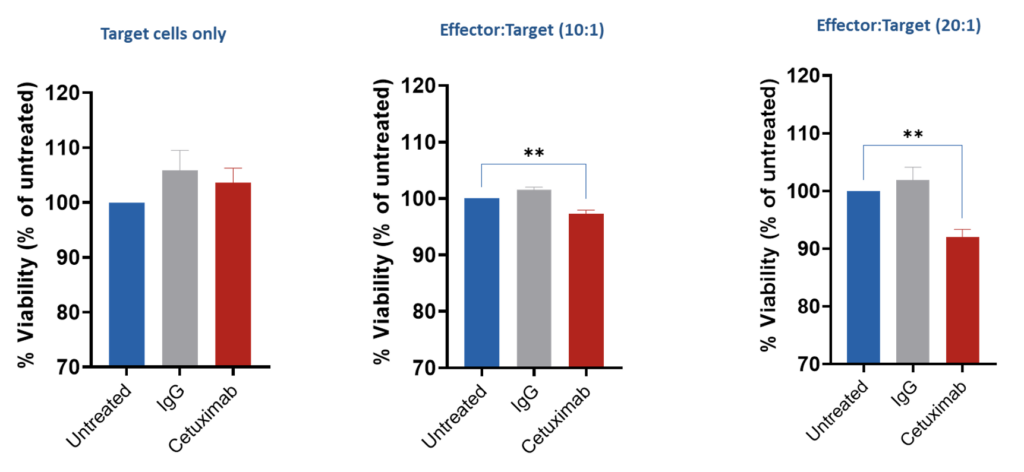
Assessment of ADCC vis GFP release – A549 (target cells)/PBMCs (effector cells) A549 (human lung adenocarcinoma) cells were co-cultured with PBMCs from a single donor for 4 hours in the presence or absence of Cetuximab (EGFR inhibitor antibody) for 4 hours to investigate the ADCC effect. CellTiterGlo assay (ATP-based viability) was performed, and results expressed as % of untreated cells.
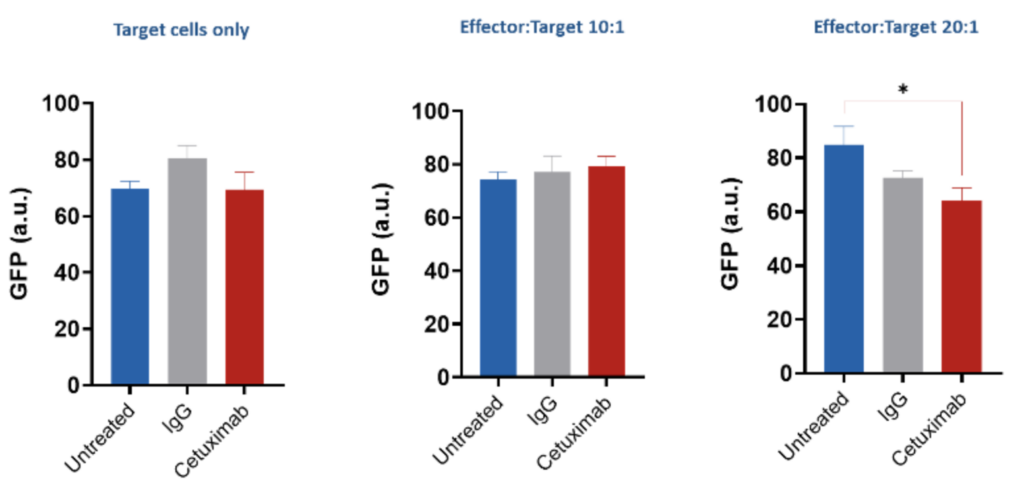
Assessment of ADCC via GFP release – HCT116 (target cells)/PBMCs(effector cells) HCT116 (human colorectal carcinoma cell) cells were co-cultured with PBMCs from a single donor for 4 hours in the presence or absence of Cetuximab (EGFR inhibitor antibody) for 4 hours to investigate the ADCC effect. Intracellular GFP levels was measured, and results expressed as relative fluorescent units.
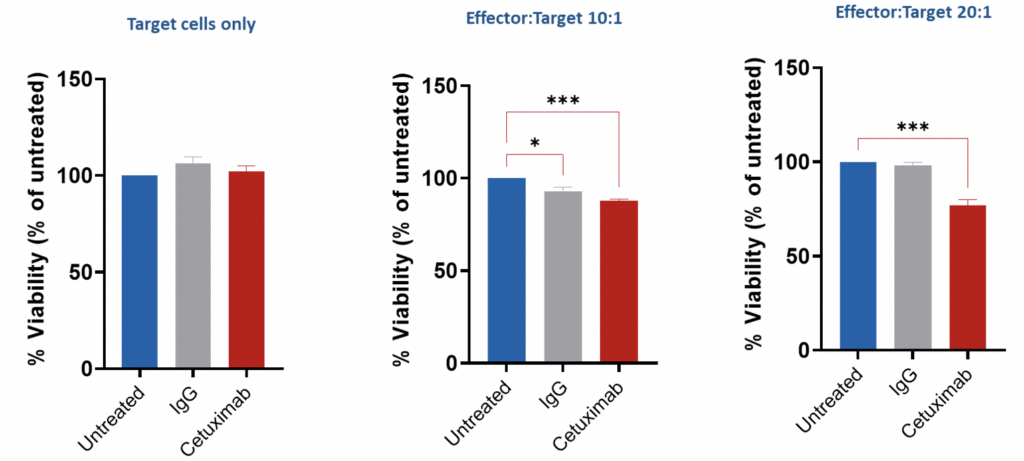
Assessment of ADCC via GFP release – HCT116 (target cells)/PBMCs(effector cells) HCT116 (human colorectal carcinoma cell) cells were co-cultured with PBMCs from a single donor for 4 hours in the presence or absence of Cetuximab (EGFR inhibitor antibody) for 4 hours to investigate the ADCC effect. CellTiterGlo assay (ATP-based viability) was performed, and results expressed as % of untreated cells.
Request a consultation with Cellomatics Biosciences today
Our experienced team of in vitro laboratory scientists will work with you to understand your project and provide a bespoke project plan with a professional, flexible service and a fast turnaround time.
To request a consultation where we can discuss your exact requirements, please contact Cellomatics Biosciences.