Toxicity Profiling
A lot of compounds, although efficacious, may fail to meet the acceptable toxicity profile. Toxicity profiling and testing is therefore an important facet of drug development. The objective is to ensure that the test substance is safe for further preclinical/clinical development.
Drug research and development costs over $145 billion every year in the US only. One of the key contributors to these costs is an unacceptable drug safety profile either detected during Phase 1 Clinical Trials (50-60%) or during Phase IV Post marketing surveillance where some severe side effects were unnoticed during the phase I clinical trial. Toxicity profiling is therefore a vital cost-saving precaution. Of note, it has been described that in 30% of the studied cases the animals showed a toxic effect on a different organ compared to human (https://www.gwern.net/docs/dnb/2000-olson.pdf).
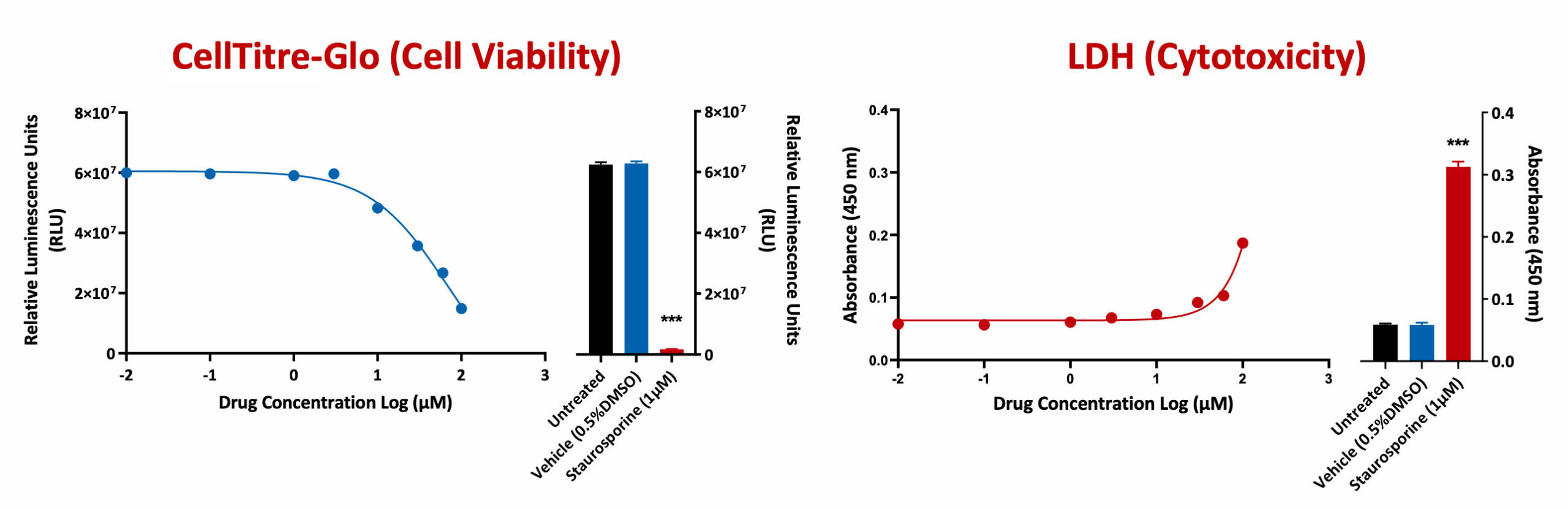
The CellTiter-Glo Viability Assay is a homogeneous method of determining the number of viable cells in culture based on quantitation of the ATP present, an indicator of metabolically active cells. The LDH-Glo™ Cytotoxicity Assay is a plate-based assay for quantifying lactate dehydrogenase (LDH) release into the culture medium upon plasma membrane damage.
A549 cells were treated with test compounds in a 8 dose response curve. Both cell viability and cytotoxicity was assessed using CellTitre-Glo and LDH respectively. Staurosporine (a positive reference control compound) showed a significant reduction in cell viability when compared to untreated or vehicle control (***p<0.001; ±SEM).
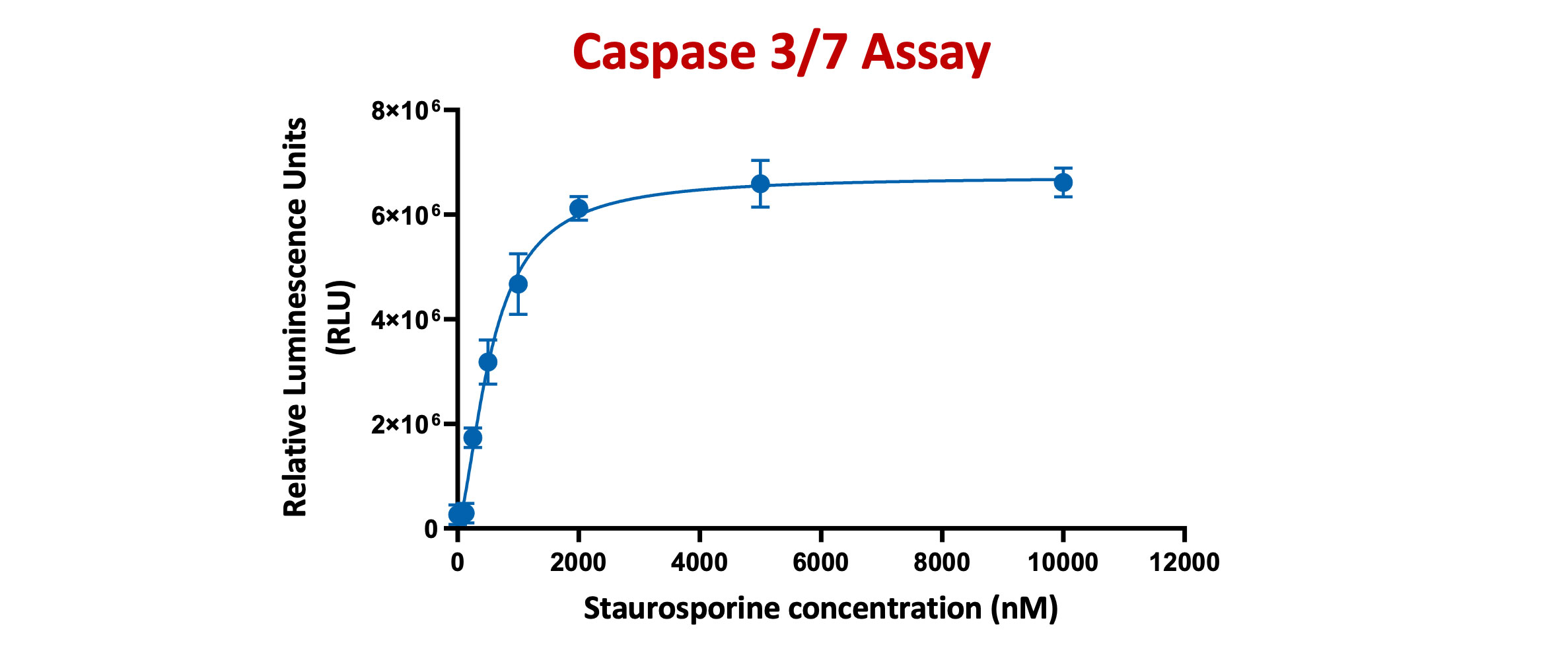
The Caspase-Glo® 3/7 Assay is a homogeneous, luminescent assay that measures caspase-3 and -7 activities. Luminescence is proportional to the amount of caspase activity present
HeLa cells were treated with Staurosporine for 4 hours at different concentrations ranging from 125nM TO 10µM. Caspase-Glo 3/7® Reagent was added directly to cells in 96-well plates and incubated at room temperature for 30 minutes before recording luminescence (RLU). Each point represents the average of 3 replicates (error bars represent ±SEM).
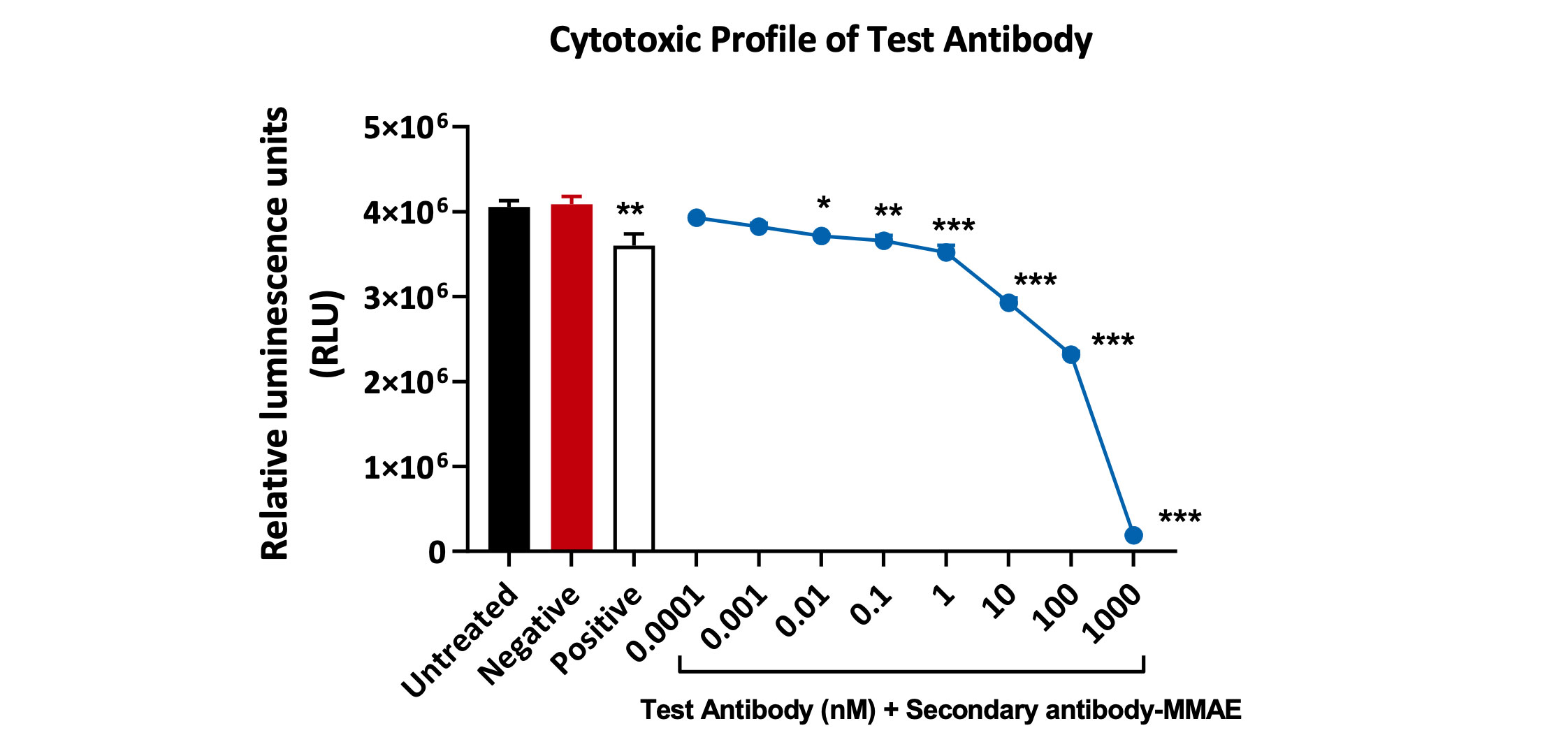
Cytotoxic profile of a test antibody (1°Ab) in the presence of 2° antibody conjugated with-MMAE toxin. Viability of Kasumi-3 cells was analysed using Cell-Titer Glo Luminescence assay. A statistically significant decrease in Kasumi-3 cell viability was observed with concentrations above 0.01 nM. Data analysed by one-way ANOVA, followed by Dunnett’s post-hoc multiple comparison test (*p<0.02; **p<0.005; ***p<0.0003, <0.0001; ±SEM).
| Acute kidney disease | Chronic kidney disease | Nephrotoxicity |
|---|---|---|
| Neutrophil gelatinase-associate lipocalin (NGAL) | Asymmetric dimethylarginine (ADMA) | N-acetyl-β-D-glucosaminidase (NAG) |
| interleukin-18 (IL18) | Neutrophil gelatinase-associate lipocalin (NGAL) | Glutathione S-transferases (GST) |
| Kidney Injury Molecule-1 (KIM1) | Kidney Injury Molecule-1 (KIM-1) | Gamma-glutamyltransferase (GGT) |
| Cystatic C (CST3) | Liver-type Fatty acid binding protein (L-FABP) | Kidney Injury Molecule-1 (KIM1) |
| Liver-type Fatty acid binding protein (L-FABP) | Lactic dehydrogenase (LDH) | |
| Insulin like growth factor binding protein (IGFBP7) | ||
| Tissue metallopeptidase inhibitor 2 (TIMP-2) |
| Human | Mouse | Rat |
|---|---|---|
| Albumin | Clusterin | Albumin |
| Clusterin | Cystatin C | Calbindin |
| Collagen IV | Epithelial growth factor | Clusterin |
| Cystatin C | Interferon gamma-induced protein 10 | Cystatin C |
| Glutathione S-transferases | Kidney Injury Molecule-1 | Glutathione S-transferases |
| Interferon gamma-induced protein 10 | Lipocalin-2 | Interferon gamma-induced protein 10 |
| Kidney Injury Molecule-1 | Osteopontin | Kidney Injury Molecule-1 |
| Lipocalin-2 | Renin | Lipocalin-2 |
| Liver-type Fatty acid binding protein | β-2-Microglobulin | Osteopontin |
| Macrophage activator protein | Tissue metallopeptidase inhibitor 2 | Tissue metallopeptidase inhibitor 2 |
| Matrix Metalloproteinases (MMP9) | Vascular Endothelial Growth Factor | Vascular Endothelial Growth Factor |
| Osteoactivin | β-2-Microglobulin (β2M) | |
| Osteopontin | Interleukin 2,6,8,10 (IL2, IL 6, IL8, IL10) | Interleukin 2,6,8,10 (IL2, IL 6, IL8, IL10) |
| Renin | Trefoil factor 3 | |
| Trefoil factor 3 | ||
| a-1-Microglobulin | ||
| Heat Shock Protein 70 (HSP70) | ||
| Interleukin 2,6,8,10 (IL2, IL 6, IL8, IL10) |
| Human | Rats |
|---|---|
| Liver-Type Arginase 1 (ARG1) | Liver-Type Arginase 1 (ARG1) |
| Malate dehydrogenase 1 (MDH1) | aspartate transaminase 1 (GOT1) |
| α-glutathione S-transferase (GSTα) | α-glutathione S-transferase (GSTα) |
| Sorbitol Dehydrogenase (SDH) | Sorbitol Dehydrogenase (SDH) |
| 5′-Nucleotidase/CD73 (5’-NT) | 5′-Nucleotidase (5′-NT/CD73). |
Request a consultation with Cellomatics Biosciences today
Our experienced team of in vitro laboratory scientists will work with you to understand your cell-based assay development needs and provide a bespoke project plan with a professional, flexible service and a fast turnaround time.
To request a consultation where we can discuss your exact requirements, please contact Cellomatics Biosciences.